CuS Thin Films Obtained by Spray Pyrolysis
Horea Iustin NAŞCU* and Violeta POPESCU
Technical University of Cluj-Napoca, Romania, http://chimie.utcluj.ro/~nascu
Abstract
The paper presents a study concerning the influence of deposition parameters (reagents, electron donors, surfactants, temperature of the substrate, number of consecutive layers, spraying rate) on the quality of CuS thin films achieved by spray pyrolysis on glass substrate, from solutions containing CuCl2·2H2O, thiourea, electron pair donors and surfactants. As electron pairs donors ammonia and triethanolamine were used and as surfactants C12 - C14 ether-sulphate, sodium lauryl-sulphate and cetyl-pyridinium bromide were used. The optimum conditions for the obtaining of optically clear, compact and adherent CuS thin films, showing different colors in reflected light (violet, yellow, brawn, green) and exhibiting in VIS and NIR light appropriate reflectance and transmittance for utilization for solar control applications were established. The film thickness falls in the range 10- 50 nm.
Keywords
copper sulphide, spray pyrolysis, thin films, solar control coatings
1. Introduction
Metal sulphides films, such as lead sulphide - PbS, cadmium sulphide - CdS or copper sulphide - CuS, metallic films of Cu or Al and nitride films - TiN, can be used as solar filters, in controlling the solar radiation, thanks to their optical properties.
Ideal, the solar control coatings must have controlled optical transmittance (Tvis = 1050%) and low reflection (Rvis < 10 %) in visible range spectra (λ = 400 - 700 nm), and a high reflection (NIR till 90 %) for wavelength bigger than 0.7 μm. Those characteristics will assure an adequate illumination of the buildings inside, and in the same time, reflecting the incident NIR radiation will stop the increase of the temperature inside the buildings, thus reducing the necessary costs for cooling [1].
The copper sulphide films deposited on glass substrate respond just to this objective
The obtaining conditions and some of the properties of CuxS films prepared by spray pyrolysis are presented in the literature [2 - 5]. We also obtained this kind of films using aqueous solutions of copper chloride - CuCl2·H2O, thiourea - SC(NH2)2 (TU), and a surfactant, to a molar ratio Cu (II):thiourea 1:5, at spraying temperature ranged between 150210°C [6].
The aim of this paper is the study of the influence of the deposition conditions on the quality of the CuS films obtained by spray pyrolypis, for control solar coatings.
2. Experimental procedure
2.1. The deposition of the film
The apparatus used to carry out the chemical spray process consists basically of a device used to atomise the spray solution and some sort of substrate heater [6]. A typical experimental setup presented by Chamberlin and Skarman [7] was used. Our setup consists of a reaction chamber foreseen to its lower part with a plate heated by electrical resistance. Standard commercial glass slides (75´25´1 mm3) were used as substrates. Substrate temperature is measured with a thermocouple. Above the substrate at variable distances (10 - 30 cm) the glass-spraying nozzle is fixed. The solution is sprayed (from a reservoir) by means of the carrier gas, incidently to the substrate. The spraying solution consists on an aqueous solution of copper chloride – CuCl2·2H2O, thiourea - SC(NH2)2 an appropriate surfactant, and sometimes complexing agents such as ammonia and triethanolamine (TEA). All substances used were analytical grade reagents. The substrate temperature was 150 - 210°C. The gas (dry air used as a carrier gas) flow rate was 6 l·min-l. The spraying time vary between 10 - 20 seconds for one layer, and the layer number between 1- 5.
2.2. Characterization of the film
The thickness of the film was established by microweighting or spectrophotometrically as described in § 3.
The VIS transmission spectra have been recorded with a Specord UV-VIS spectrometer and those of NIR transmission and reflection with a UR-20 Clad Zeiss Jena spectrophotometer. The absorbance was determined with a photocolorimeter FEK -M.
3. Results and discussions
3.1. The determination of film thickness
The thickness of the films was determined using a micro gravimetrical method. The films were deposited on clean glass slides whom mass was previously determined. After the deposition the substrates it was again weighted, determining the quantity of deposited CuS. Measuring the surface of the deposited film, taking account of CuS specific weight of the film, thickness was determined using the relation:
![]()
![]() ×104 mm
×104 mm
where S is the surface of the film [cm2], mCuS is the quantity of deposited cooper sulfide, and r is the specific weight of CuS.
For the samples with known thickness the absorbance was determined as a function of the thickness of the film. The calibration curve for CuS film thickness determination is presented in fig. 1.
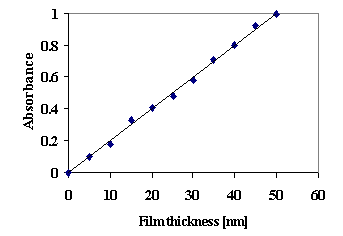
Fig. 1. Calibration curve on the spectrophotometric determination of CuS film thickness
3.2. The influence of the concentration
Suitable copper salts for spraying solutions are copper chloride - CuCl2·H2O, and copper acetate - Cu(CH3COO)2·3H2O. Better results regarding the uniformity and the quality of the films were obtained using copper chloride. Regarding the quantity of thiourea from solution, in the most of the cases a molar ratio Cu:thiourea of 1:1 was used, but experimental determinations were made also for 1:2, 1:4, 1:5, and 1:8 ratio.
In agreement with Ugai et all data [2], the deposition of copper sulphide films, by spray pyrolysis using aqueous solutions containing only Cu (II) ions and thiourea, takes place through the formation of an intermediary complex. The intermediary complex starts to forms in aqueous solution before spraying. In the same time, before spraying takes place the reduction of Cu (II) to Cu (I). The complex which is formed correspond to the following formula Cu[SC(NH2)+Cl-. Nair et al [8] mentioned also the formation of a Cu(I) complex with thiourea corresponding to the formula Cu4[SC(NH2)2]64+. In this case the obtained films correspond to Cu2S formula.
Table 1. The influence of reagents concentration (Tu, TEA, cety-pyridinium bromide (BCP)), pulverization rate V of the solution, the substrate temperature, the pulverization time t, T VIS. and the thickness h of the film
|
Sample No. |
The composition of the solution |
Cu:TU |
h [nm] |
Spraying conditions |
TVIS [%] |
Observations |
|||
|
T [°C] |
V [ml/min] |
t [sec] |
No. of layers |
||||||
|
450 |
CuCl2·2H2O - 0.01M TU - 0.04 M |
1:4 |
10 |
200 |
20 |
10 |
1 |
76 |
Uniform, yellow, clear film |
|
451 |
CuCl2·2H2O - 0.01M TU - 0.05 M |
1:5 |
8 |
200 |
12 |
20 |
1 |
83 |
Uniform, yellow, clear film |
|
452 |
CuCl2·2H2O - 0.01M BCP 10-2% |
1:4 |
17 |
200 |
20 |
50 |
5 |
58 |
Uniform, brown, clear film |
|
472 |
CuCl2·2H2O - 0.01M TU - 0.01 M BCP 10-2% |
1:1 |
10 |
200 |
15 |
20 |
2 |
76 |
Uniform, yellow, clear film |
|
473 |
CuCl2·2H2O - 0.01M TU - 0.01 M BCP 10-2% |
1:1 |
28 |
200 |
10 |
15 |
4 |
42 |
Uniform, brown, clear film |
|
475 |
CuCl2·2H2O - 0.01M TEA - 0.02M TU - 0.01 M BCP 10-2% |
1:2 |
22 |
200 |
6 |
10 |
7 |
58 |
Uniform, brown, clear film |
In our case, using in addition a surfactant seems that the reducing reaction of Cu(II) to Cu (I) do not take place, because the deposited film consists only on CuS (according to the chemical analysis and XRD) [6].
The data regarding the influence of reagents concentration on the film formation by spray pyrolysis are presented in table 1.
For a molar ratio Cu:TU of 1:8 the obtained films are not continuous, being formed by black islands. The optimum molar ratio Cu:TU of 1:4-1:5 was established taking into consideration the uniformity of the film and the stability of the spraying solution. The solution is stable only a limited period of time after those complexes are formed (as precipitates) and solutions can not be used any more for film deposition.
3.3. The influence of surfactants
The surfactants added to the spraying solutions increases the stability of those solutions, and the uniformity of the deposited film.
As surfactants, ethersulphate C12-14 (ES), sodium lauryl sulphate CH3(CH2)11OSO3Na (the both of then being anionic) and cetyl-pyridinium bromide C2lH38NBr (cationic agent) were used. Using sodium lauryl sulphate the solution becomes turbid; that was the reason-the use of it was excluded. 4·10-2 - 1.64·10-1 amounts of ES and 1-2∙10-1 cetyl-pyridinium bromide added to the spraying solutions leads to a good stability of the spraying solution as well as to the formation of quality films (at Cu(II) concentrations of about 0.01 M).
3.4. The influence of complexing agents
When working with a molar ratio Cu:TU of 1:1, the using of some ligands, such as ammonia or TEA, was necessary. In the case of ammonia, a larger amount, as compared with the concentration of Cu (II), was necessary in order to obtain a stable spraying solution. Using TEA, the quantity was 10-15 times smaller than in the case of ammonia. The ratio Cu:TEA vary between 15-24. By increasing this ratio, the stability of solution increases too, especially at smaller Cu (II) concentrations. Beside this, the quality of the obtained films from solutions containing TEA was better.
3.5. The influence of the pulverization rate and time
On the basis of our experiments, the best films concerning uniformity, clarity and transparency were obtained using small pulverization rate (10-40 ml∙min-l) for short pulverization time. The use of higher rates, leads to the following inconvenients: the cooling of the substrate (and implicit the impossibility of maintaining a constant temperature) as well as to the appearance, in some of the cases, of bigger drops and consequently to ununiform films.
Optimum spraying rates of 25-35 ml∙min-1 for short pulverization time (10-20 seconds) were established. For reaching the desired thickness more than one layer must be deposited. One minute between subsequently pulverisation was necessary in order to maintain constant temperature. The variation of the thickness of the film with the deposition time is linear (fig.2).
3.6. The influence of the substrate temperature
With the increases of the substrate temperature, the quality of the formed films was improved. The experiments were performed at temperatures in the range 150-210°C. The best results were obtained at 200-210°C. This range of temperature was chosen because CuS is reported to decompose at ≈ 220°C [9]. The decomposition of the films at 220°C was observed by the conversion of the film to a nearly transparent one.
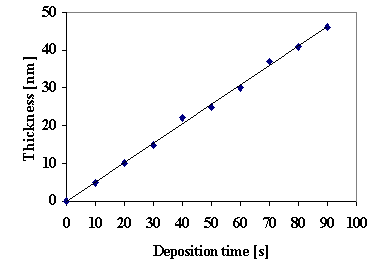
Fig. 2. Dependence of the thickness of CuS films on the deposition time
The optimum distance between the spraying nozzle and the substrate of about 25-30 cm was established.
3.7. Optical properties of the films
In fig. 3 the transmission spectra of CuS films having different numbers of layers deposited are presented. In these conditions maximum NIR reflection, of about 25 %, was obtained.
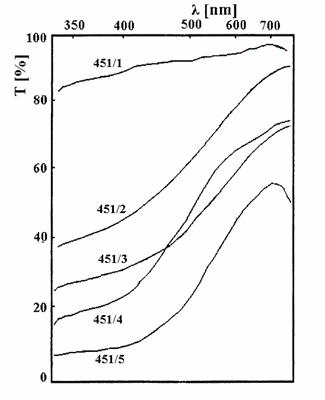
Fig. 3. The transmission spectra of CuS films
(451/1 - one layer; 451/2 - 3 layers; 451/3 - 4 layers; 451/4 - 5 layers; 451/5 - 9 layers)
4. Conclusions
On the basis of our studies, optimum conditions for spray pyrolysis deposition of CuS films, for solar control applications were established. Solution containing CuCl2∙2H2O 0.016 M, thiourea (TV) 0.08 M and cetyl-pyridinium bromide was used. The substrate temperature was 200-210°C. The solution flow rate was 25-35 ml∙min-1 and carrier gas flow rate (air) was 6 l∙min-1. The nozzle substrate distance falls between 25-30 cm. The solution was sprayed on the substrate for 10 s. After 1-2 minutes the cycle was repeated until the desired thickness was achieved. The film thickness lies between 10-50 nm.
Acknowledgments
This work was supported by MEC under CNCSIS grant nr. 33531/2002.
References
1. Naşcu C., Ionescu (Popescu) V., and Pop I., Rev. Chim., (Bucharest) 49, 1998, p. 535.
2. Ugai Ya.A., Semenov V.N., and Averbach R.M., Zh. Neorg. Khim., 26, 1981, p. 272.
3. Vedel J., Cowache P., and Decraoni M., Rev. Phys. Appl, 15, 1980, p. 1521.
4. Bursuc I., Zet Gh., Gherghel M., Roşca I., Gherasimescu R. and Mihai I., Bul. Lnst. Polit. Iaşi, 29, 1983, p. 79.
5. Soriano L., Leon M. and Arjona P., Sol. Enefigy Mater., 12, 1985, p. 149.
6. Naşcu C., Pop I., Ionescu (Popescu) V., Indrea E. and Bratu I., Matt. Lett., 32, 1997, p. 73.
7. Chamberlin R.R. and Skarman J.S., J. Electrochem. Soc., 113, 1996, p. 86.
8. Nair M.T.S. and Nair P.K., Semicond. Sci. Technol., 4, 1989, p. 191.
9. Hanes A., Kenl M., Mieroiu E., Sandulescu D. and Zaharia M., Manualul lnginerului Chimist, Vol I, Editura Tehnica, Bucharest, 1972.