Production and Characterisation of Zeolite from
Ahako Clay in Kogi State, Nigeria
A. S. KOVO and M. O. EDOGA
Department of Chemical Engineering, Federal University of Technology, Minna, Nigeria
Abstract
Zeolite, a multi-purpose material normally sourced from clay is usually for many Engineering applications. However, it is only those Zeolite that are technically well prepared that can give optimum performance during application. Obtaining a constant route for development of Zeolite in Nigeria will be a welcome development. In this study, therefore, emphasis was placed on the development of various type of Zeolite. In attempt to synthesize the Zeolite samples, 1kg of Ahako clay was beneficiated by soaking it in deionised water for four days during which the mixture was vigorously agitated. Subsequently, the mixture was sieved with a minus 60 mesh (Tyler fraction or Micro sphere) of which the screened mixture was treated with 1 litre of sodium bicarbonate solution and sodium hexameta-phosphate in the ratio of 1:4 primarily for deflocculation or dispersion. The washed clay was dried in an oven set at 45oc for two days after which it was ground to fine powder.
The beneficiated clay was calcined at 600°C in furnace (model 6H-85IR) for two hours. Subsequently, the calcined clay was formulated into various samples by properly incorporation sodium hydroxide (Noah), Silica gel and deionised water or weight basis.
The results of the XRF showed that the crude and treated clay (calcined clay) contained 73.18% by weight of Sio2 and 14.5% by weight of Al2O3 and 73.20% by weight of SiO2, 7.48 by weight of Al2O3.
The developed Zeolite sample should feature in their spectra (IR) that are consistent with Zeolite D as obtained in sample 1 (freshly prepared), Zeolite N-A as obtained in sample II cum sample IV after aging time of 48hours and Zeolite A as obtained in sample III.
Keywords
Ahako Clay, Zeolite, Deflocculation, Infrared spectroscopy (IR), X-ray fluorescence (XRF), Beneficiation, Calcinations
1. Introduction
Zeolite is usually synthesized under hydrothermal conditions from solutions of sodium aluminate. Sodium silicate or typical of those found in the earth’s crust where some Zeolite are found naturally [1]. The first claim to a synthetic Zeolite (levnite) appeared to be that of Sainte-Claire Deville in 1862 [2].
The zeal to develop Zeolite was sustained until 1948 when Milton and Associate reported the existence of synthesised Zeolite which never existed before as minerals [3]. One remarkably discovery from the work of Don Breck (1974) was the revelation of the possibility of synthesizing Zeolite at very mild temperature ranging from room temperature and above.
Kaolin clay group, which is represented chemically as Al2O3SiO2∙2H2O, is an important material used in the synthesis of Zeolite. Kaolin clay is normally converted to metakaolin by a process known as calcinations (thermal treatment) at a temperature of about 600°C. Metakaolin is generally known to be a defect phase in which the tetrahedral silica layer of the original structure are largely retained even though they still exist some controversies about the true nature of metakaolin phase. Metakaolin is more reactive and can easily be reacted by either acid or alkalis [5, 6]. Because of availability and low cost of clay, most industrial production processes presently utilize Kaolin clay in the manufacture of Zeolite, therefore the aim of this work is to look at the suitability of Ahako clay for the synthesis of Zeolite and therefore characterise it [7].
2. Experiment
Materials and chemicals used to produce and characterise Zeolite from Ahako clay include silica gel, sodium hydroxide and Deionised water.
Instruments used include common glassware, electrical furnace, oven, X-ray fluorescence (XRF) and infrared (IR) spectrophotometer.
Procedures
One kilogram of kaolin clay was procured from Ahako village in Kogi State Nigeria and was soaked in deionised water for four days during which it was vigorously mixed using a magnetic stirrer.
A minus 60 mesh (Tyler) fraction was obtained by screening 1 litre of 1:4 sodium bicarbonate and sodium hexa-metaphosphate solution was added to the clay sample for process of deflocculation, the products of deflocculation were allowed to stand for eight hours, after which it was decanted (table 1). The excess deflocculant noticed for its removal. The clay recovered was then dried at 45°C for two days in an oven. The dried was calcined in a furnace at a temperature of 600°C for two hours.
Table 1. Gel of varying compositions
|
Sample |
I |
II |
III |
IV |
|
Calcined clay |
5 |
9 |
10 |
10 |
|
NaOH |
9 |
5 |
9 |
15 |
|
Silica gel |
10 |
10 |
5 |
9 |
|
H2O |
5 |
15 |
15 |
5 |
Each of the sample was left to age for period ranging from 24 hours to 48 hours at room temperature. The mixture was occasionally stirred to increase homogeneity of the mixture.
Characterization of the Zeolite samples were reformed using infrared spectroscopy (ATI Mattson genesis series) Fourier transform infrared spectrometer for freshly prepared zeolite samples, zeolite sample aged for 24 hours and zeolite sample aged for 48 hours. The chemical composition of both the crude clay and treated clay (calcined clay) was determined using X-ray fluorescence.
3. Results and Discussions
The chemical analysis of both the crude (untreated) and calcined (treated) clay are in table 2. The result shows that the percentage elemental composition of the untreated clay is as follows: SiO2-73.12, Al2O3-14.53, and Fe2O3-1.35. The amount of SiO2 and Al2O3 present in the untreated clay (Ahako) give a ratio of SiO2/Al2O3 as 73.18/14.53, which is greater than 1. This is in accordance with the Breck (1974) specification for clays that can be used for Zeolite synthesis. The conformity of the Ahako clay with the Breck (1974) specification invariably implies that the Ahako clay can be used for Zeolite formation.
Table 2. Chemical analysis of Ahako clay
|
|
SiO2 |
Al2O3 |
Fe2O3 |
CaO |
MgO |
SO2 |
N2O |
LOI |
CaCO3 |
|
Crude Kaolin |
73.18 |
14.53 |
0.7 |
1.35 |
6.81 |
0.08 |
- |
5.01 |
2.4 |
|
Calcined Kaolin |
73.22 |
7.48 |
0.27 |
1.12 |
0.65 |
6.87 |
0.25 |
1.2 |
2.2. |
Figures 1-5 give the infrared spectra of calcined and other Zeolite samples. The IR spectrum of the freshly prepared Zeolite sample is consistent with that of Zeolite D as given in by Breck (1974). Figure 3 shows that vibrational changes took place as a result of the ageing of Zeolite sample II, there is a clear shift in the transmitting of aged Zeolite sample compared with the freshly prepared Zeolite. This shift exhibited by the aged Zeolite is related to vibration of the internal tetrahedron structure (primary unit Al, SiO) known as asymmetrical stretch and symmetrical stretch respectively. The IR spectrum of the aged Zeolite spectra is found to be consistent with that of Zeolite N-A [4]. The deviation of peaks recorded from the experimental work as compared with literature might be due probably to the level of purity of the sample and the fact that the preferred method of analysis was not used. There is a similar shift in the vibration of Zeolite Sample III as a result of ageing of the freshly prepared Sample as shown in fig. 4.
The internal T-O vibrations of the freshly prepared Zeolite sample III (970.39, 721.82 and 461cm-1) were found to change after ageing process. The changes can be attributed to the possible mechanism leading to the formation of Zeolite. The peak recorded for sample III is consistent with the peak value for Zeolite A. Figure 5 shows the spectra of freshly prepared gel and gels obtained after ageing. Fully-grown Zeolite N-A was obtained after 48hours of ageing.
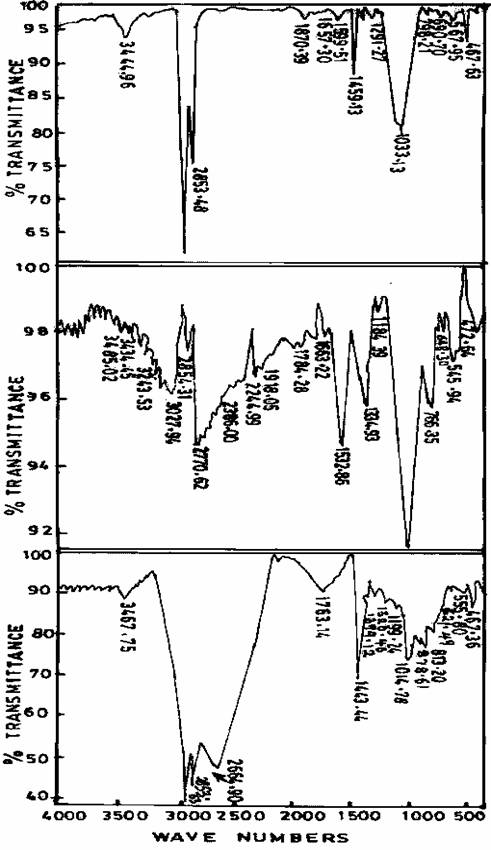
Figure 1. Infrared spectra of freshly prepared, Gel aged of 24hrs, and Gel aged for 48hrsof Zeolite N-A sample
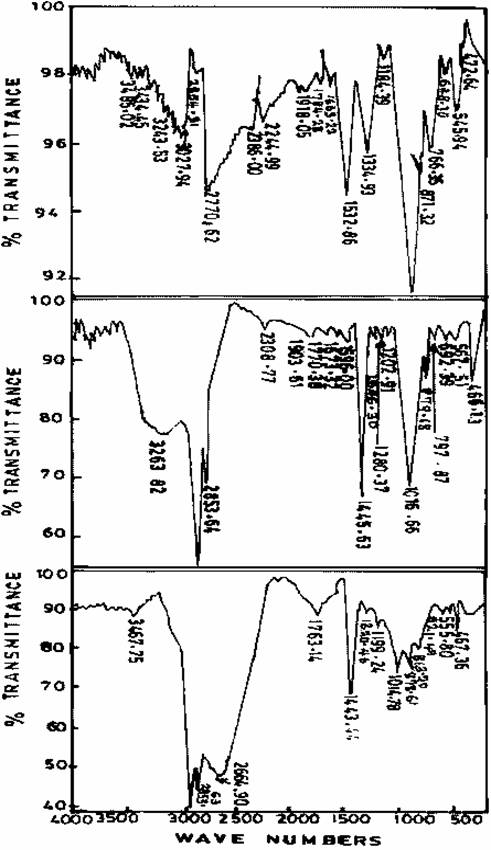
Figure 2. Infrared spectra of freshly prepared, Gel aged of 24hrs, and Gel aged for 48hrs of Zeolite sample I
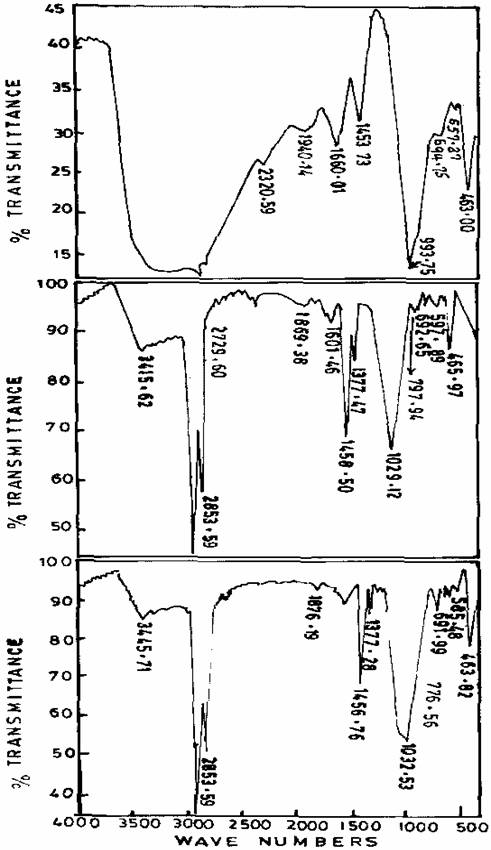
Figure 3. Infrared spectra of freshly prepared, Gel aged of 24hrs, and Gel aged for 48hrs of Zeolite sample II
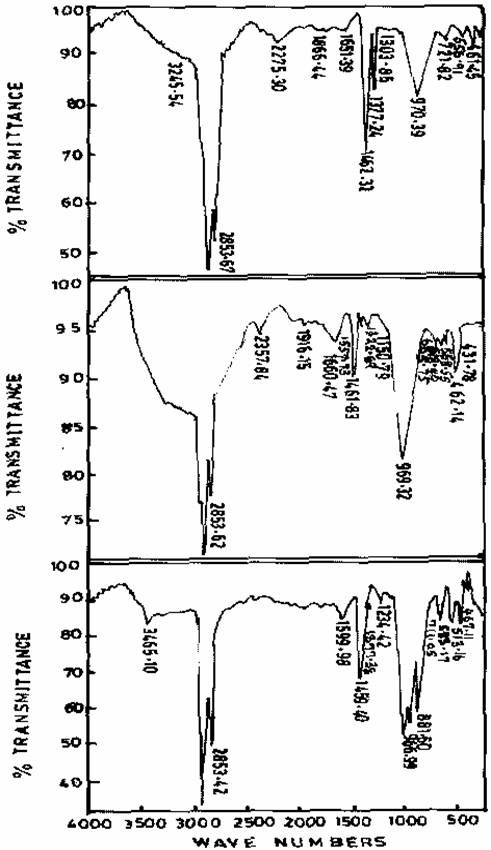
Figure 4. Infrared spectra of freshly prepared, Gel aged of 24hrs, and Gel aged for 48hrs of Zeolite sample III
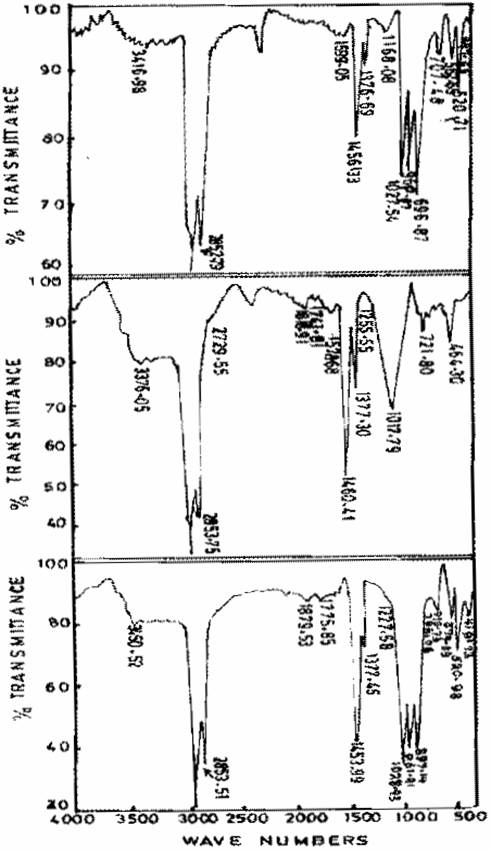
Figure 5. Infrared spectra of freshly prepared, Gel aged of 24hrs, and Gel aged for 48hrs of Zeolite sample IV
4. Conclusion
This paper has shown that Zeolite can be synthesised from large deposit of kaolin in Ahako village near Lokoja, Kogi State. The result of the chemical analysis carried on the raw kaolin showed that it contained substantial amount of silicate and aluminates, which quantified it to be used in the formulation of Zeolite. The raw kaolin contains 73.10% SiO2 and 14.53% Al2O3 giving a Si/Al ratio that is greater, which is a basic prerequisite for the synthesis of Zeolite. The result of infrared spectroscopic has proven its importance in the monitoring of the time formation of Zeolite. It can be concluded that Ahako clay is a potential source for synthesis of Zeolite A, D and N-A in Nigeria.
References
[1] Aiello R. M. S. et al., Zeolite formation from synthetic and natural glasses, Advances in chemistry, series 101, 1971, p. 1-62
[2] Barrer R. M., Hydrothermal chemistry of Zeolite, Academic Press, London, 1982, p. 49, 52, and 150.
[3] Bekkum H .V. et al., Introduction to Zeolite science and practice, Elsevier, Amsterdam, 1991, p. 126 and 135.
[4] Breck D. W., Zeolite molecular sieve structure, chemistry and uses, Wiley InterScience, New York, 1974, p. 262-275, 314, and 245-300.
[5] Flanigen E. M., The word and work of Don Breck, Proceeding of the Sixth International Zeolite Conference, 1983, p. 3-16.
[6] De Luca et al., Progress in zeolite and microporous materials, Elsevier, Amsterdam, 1997, p. 325.
[7] Zhdavous S. P., Some problems of Zeolite crystallization, Advances in Chemistry Series 101, 1971, p. 20-43.