Effect of Water Quality on the Performance of Boiler in Nigerian Petroleum Industry
J. O. ODIGURE, A. S. ABDULKAREEM, and E. T. ASUQUO
Chemical Engineering Department, Federal University of Technology, Minna, Nigeria
Abstract
This work investigates quality of water used in boilers of Refinery Company in Nigeria. The results shows that the quality of water fed to boilers are off specification. Low water quality used in boilers led to frequent failure of the boilers as a result of tube rupture. This has resulted into low capacity utilization and loss of processing fees. The poor performance of the boiler feed treatment plant is attributable to the deplorable condition of water intake plant, raw water treatment, demineralization plant, change in raw water quality and non-functioning of the polisher unit.
Keywords
Water quality, Demineralization, Water treatment
Introduction
A boiler is a closed vessel in which water under pressure is transformed into steam by the application of heat. In the boiler furnace, the chemical energy in the fuel is converted into heat, and it is the function of the boiler to transfer this heat to the contained water in the most efficient manner. The boiler should also be designed to generate high quality of steam for plant use (Abdulrahamn, 2001). A boiler must be designed to absorb maximum amount of heat released in the process of combustion. This heat is transferred to the boiler water through radiation, conduction and convection. The relative percentage of each is dependent upon the type of boiler, the designed heat transfer surface, and fuel (Abdulrahman, 2001). Two principal types of boilers are used for industrial applications: fire tube and water tubes boilers. In the fired tube boilers, products of combustion pass through the tubes, which are surrounded by water. In the water tube boilers, products of combustion pass around the tubes containing water. The tubes are interconnected to common channels or headers and eventually to a steam outlet for distribution to the plant system (Brockman, 1988). The refinery industry has five boilers. These produce the various types of steam needed for refinery processes. Steam is also used as a driver for all the turbine equipment in the refinery. The boiler feed water plant otherwise known as the ion exchange or demineralizer plant supplies water to the boilers. The boiler house or steam generation facility within any plant is frequently referred to as the heart of the plant. The unit is usually shunt down for maintenance to remove all substances in the boiler feed water that will be injurious to the boilers. Examples of such substances are positively charged calcium, potassium, sodium, magnesium, aluminum, zinc, iron, lead, and copper. On the other hand, there are negatively charged ions like sulphates, carbonates, bicarbonates, silica, chlorides and fluorides. Another dangerous substance that must be removed is the oxygen (Culp, 1974). This is done in the de - aerator that is located after the ion exchanger. The flow diagram of the boiler is shown in figure 1:
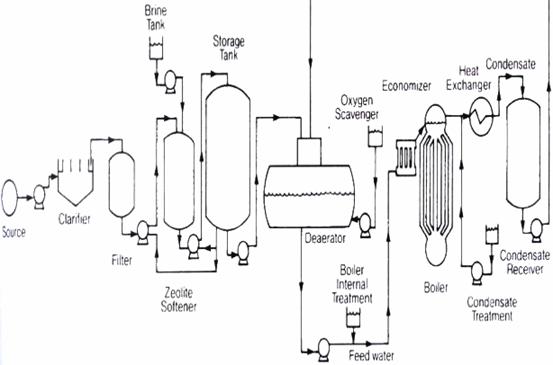
Figure 1. Boiler plant flow diagram
This write - up is aimed at carry out performance evaluation on the boiler used in the refinery. This could be achieved via realization of the following objectives:
· Carry out analysis on the boiler feed water;
· Compare the analysis results with the standard in order to evaluate the performance of the treatment plant;
· Investigate how unscheduled shutdown due tube failures from excessive deposition and severe energy losses due to deposits retarding heat transfer in the critical areas of the boiler can be prevented.
Water quality
Centralized water supp.ly systems are based on the use of surface and underground water sources and the properties of water are fundamental to the treatment processes. Water in is pure form has the following properties (Edem, 2002): it is clear and odorless, it freezes at 0°C and 1 bar, it boils at 100°C at a pressure of 760mm Hg, its maximum density is 1g/m3 at 4°C and it is neutral to litmus. However, composition of water in surface water sources (rivers, lakes, water storage basins, seas etc) is determined by many climatic and geomorphologic factors conditions of soil geology and hydro - ameliorative measure. The composition of underground (interlayer, artesian, karst etc) water depends on the condition of their formation (Nikoladze, 1996). Water is said to be polluted when it is unfit for the purpose for which it is intended. This is due to the presence of physical substances and biological pathogens that make water unfit for human consumption (Sunday, 2003). Many of the inorganic cations that we find objectionable in surface and ground water come from natural sources. Water coming in contact with limestone (CaCO3) or dolomite (CaCO3∙MgCO3) picks up calcium ions and magnesium ions which makes it too hard for many households and industrial uses. Underground deposits of iron compounds are responsible for the presence of irons ions in certain ground waters. This ion reacts with hot water to form hydrated ferric hydroxide, which deposits as a brown stain on bathtubs and clothing (Sunday, 2003). Another natural pollutant is silt, which consists of suspended minerals particles resulting from land erosion and other dissolved solids. This is the primary cause of the turbidity of water. Alga, plankton and other aquatic plants are also natural sources of water p[pollution. The discharge of sewage and industrial effluents into natural sources of water are the most serious causes of water pollution. The importance of wastewater as a vehicle for the spread of diseases has long been recognized. Researches have shown that the concentrations of the metals in fishes are higher where wastewater from industries is discharged into river (Thomas, 1987).
Ion Exchange
The mobile hydrated ions of solid are exchanged equivalent to equivalent with ions of same charge in the solution in this chemical reaction. The solid has an open fish net like structure and the mobile ions of electrically neutralize charge or potentially charged groups attached to the solid matrix, called the ion exchanger. Cation exchange occurs when the mobile, positively charged cation fixed to the negatively charged fixed group of the ion exchanger, exchanges for another cation in the solution. Likewise, anion exchange occurs when mobile negatively anion attached to the positively charged fixed group on the ion exchange resin is exchanged for another anion in the solution (Walter, 1981). Sodium and Hydrogen cation exchange process are the methods employed in the petroleum industry.
Sodium cation exchange process is the most widely method for softening water. During the softening process, calcium and magnesium ions are removed from hard water by cation exchange from for sodium ions. When exchange resin has essentially removed the calcium and magnesium ion to the limit capacity, the resin is regenerated to sodium form with salt solution in the pH range of 6 to 8. Most high capacity ion - exchange resins are silicates, organic ion exchanger made from coal, lignite and peat are also used. The reaction for softening is shown in the equations below (Walter, 1981):
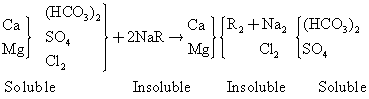 (1)
(1)
where R represent the cation exchanger radical. The regeneration reaction is as shown below:
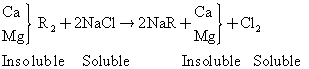 (2)
(2)
In cases where very hard bicarbonate water is encountered, it is often desirable to treat the water first by the lime process, followed by cation exchange. The lime process reduces dissolved solid by precipitating calcium carbonate and magnesium hydroxide from the water.
Hydrogen cation exchange process ids just like the sodium cation exchange process except that the exchange resins contain an exchangeable hydrogen ion and can be employed to remove all cation. The reaction is as follows:
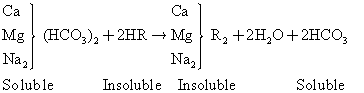 (3)
(3)
The rein is regenerated using sulfuric acid and it is most widely used because it is very economical.
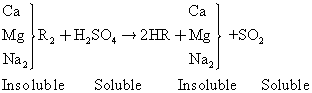 (4)
(4)
Methodology
Experimental analyses were carried out on the water feed into the ion - exchange plant and the water feed into the boiler from the ion - exchange resin plant to determine the following parameters: pH, electric conductivity, total solid, organic matter. Total dissolve solid, suspended solid, total iron, total cation (as CaCO3), Ca++ and Mg+(as CaCO3), Na++ and K+ (as CaCO3), NH4+, total anion, HCO3-, NO3-, SiO2, and CO2.
Results and Discussions
Boiler is designed to generate high quality steam for plant use. Boiler absorb maximum amount of heat released in the process of combustion, which is transferred to the content of boiler (water) to generate steam. For better performance of the boiler, the water feed into boiler must be treated to remove foreign materials present in the water.
Results of analysis carried out on boilers feed water before and after treatment in the ion exchanging plant is recorded with the design values and presented in table 1 and table 2 respectively.
Table 1. Experimental results of feed water to the ion - exchange plant
|
Parameters \ Values |
Experimental |
Design |
|
pH |
7.8 |
6.8 -7.5 |
|
Temperature (°C) |
23.8 |
22 -27 |
|
Electric conductivity (mW/cm) |
137 |
110 |
|
Total solid (ppm) |
590 |
210 - 520 |
|
Turbidity (Degree) |
6 |
1 |
|
Organic matter (ppm) |
42 |
6 |
|
Free Cl2 (ppm) |
0.6 |
0 |
|
Total dissolved solid (ppm) |
350 |
203 - 315 |
|
Suspended solid (ppm) |
20 |
5 |
|
Free Fe (ppm) |
4 |
0.3 |
|
Total cation (ppm as CaCO3) |
170 |
101.2 |
|
Ca++ and Mg+ (ppm) |
45 |
40.8 |
|
Na++ and K+ (ppm) |
51 |
59.4 |
|
NH4+ (ppm) |
3 |
1 |
|
Total anion (ppm as CaCO3) |
180 |
105.1 |
|
HCO3- (ppm) |
70 |
59.4 |
|
SO42- (ppm) |
9.2 |
5.1 |
|
NO3- (ppm) |
7.1 |
3.2 |
|
SIO2 (ppm) |
13 |
1.7 |
|
CO2 (ppm) |
4.2 |
2.2 |
|
Cl- (ppm) |
20 |
32.1 |
Design Values are the values obtained when the plant was commissioned while the experimental values are the values obtained on the boiler feed water and the boiler treated water. Table1 indicates that the design values are different from the experimental values. This indicates that the ion - exchange plant feed water is not properly treated before it is fed into the plant. It could be observed from table 1 that pH of feed water is 7.8 which does not conform to the design values of 6.8 - 7.5. This has adverse effect on upstream treatment processes. Also the silica, total iron, suspended solid, total cation design values of the boiler are 1.7ppm, 0.3ppm, 5ppm and 10.2ppm respectively while the experimental values are 13ppm, 4ppm, 20ppm and 170ppm as CaCO3 respectively. The silica, total iron, suspended solid and total cation design values are <0.17ppm, <0.1ppm, <1.0ppm and <2.0ppm respectively, while the experimental values are 1.7ppm, 0.2ppm, 3ppm and 90.2ppm respectively.
Table 2. Experimental results of treated water from ion - exchange resin to the boiler
|
Parameters \ Values |
Experimental |
Design |
|
pH |
8.5 |
7 -8 |
|
Electric conductivity (mW/cm) |
4 |
≤ 10 |
|
Total solid (ppm) |
80 |
≤ 5.0 |
|
Organic matter (ppm) |
3 |
1 |
|
Total dissolved solid (ppm) |
200 |
< 5.0 |
|
Suspended solid (ppm) |
3 |
< 1.0 |
|
Total Iron Fe (ppm) |
0.2 |
< 0.1 |
|
Total cation (ppm as CaCO3) |
90.2 |
≤ 2.0 |
|
Ca++ and Mg+ (ppm) |
25.8 |
0 |
|
Na++ and K+ (ppm) |
39.4 |
2.0 |
|
NH4+ (ppm) |
1 |
0 |
|
Total anion (ppm as CaCO3) |
95.1 |
≤ 0.1278 |
|
HCO3- (ppm) |
39.4 |
0 |
|
SO42- (ppm) |
3.1 |
0 |
|
NO3- (ppm) |
2.2 |
0 |
|
SiO2 (ppm) |
1.7 |
< 0.17 |
|
CO2 (ppm) |
0.73 |
0 |
Table 3. Boiler Feed water condition on failure
|
Serial Number |
Boiler feed water condition |
|
|
pH |
SiO2 |
|
|
1 |
10.61 |
0.52 |
|
2 |
10.49 |
0.92 |
|
3 |
10.49 |
- |
|
4 |
10.93 |
0.52 |
|
5 |
10.98 |
0.57 |
|
6 |
10.61 |
0.5 |
|
7 |
9.05 |
1.5 |
The difference in values between the design and experimental values could be attributed to the fact that the exchange capacities of both weak and strong cation and anion resin have reduced considerably from the specified values. This is supported by the fact the resin regeneration is done thrice as against the design of once per day. Also poor treatment of feed water and deplorable condition of the facilities of the ion - exchange plant also, contributed to the variation between the design and experimental values. Effect of poorly treated water used in the boiler for steam production is manifested through scaling and eventual failure of the boiler as a result of tube rupture as shown in table 3 of results. This has lead to loss of revenue, low capacity utilization and loss of processing fees.
Conclusion
Based on the results obtained it could be concluded that the performance of the boiler is low and it is not working in conformity with the design. The boiler failures observed were partly due to the poor boiler feed water quality, which, was traced to very deplorable condition of the water feed into the boiler.
References
[1] Abdulrahaman M., Evaluation and modeling of effluent from Wuye wastewater treatment plant, M. Eng. Thesis, Federal University of Technology, Minna, Nigeria, 2001, p. 35-39.
[2] Brockman J. L., Kulshreshtha S. N., The residential demand for water in Saskatchewan: A problem of price specification with block rate schedule, International Water Resources Agency, USA, 1988, p. 477-486.
[3] Culp G. L. & R. F., New concept in water purification, Van Reinhold Company, New- York, 1974.
[4] Delwyn D., Fresh water, International Learning Systems, London, 1967, p. 30-35.
[5] Fair S., Water supply and Wastewater Disposal, John Wiley and Sons, New-York, 1979, p. 154-156.
[6] Chyoda C., Mechanical catalogue for utilities facilities, 1980, p. 1-30.
[7] Edem E. A., P.P.U. equipment Maintenance and Consumption in K.R.P.C. Nigeria, 2002, p. 60-76.
[8] Funtua M. M., Factors militating against and efficient operation of KRPC utilities plants, 2000, p. 45-54.
[9] Fredericks W. P., Water quality and treatment, R. R. Donnelley and Sons Company, USA., 1990, p. 158-160.
[10] Walter L., Handbook of water purification, Ellis Horwood, New York, 1987, p. 85-90.
[11] Nikoladze G., Mints A. K., Water Treatment for public and industrial supply, MIR Publishers, Moscow, 1989, p. 65-72.