Collaborative Influence of Zinc Oxide and Triethylene Amine on the Corrosion Behavior of Mild Steel in Hydrogen Cyanide Environment
Muhammed Olawale Hakeem AMUDA1, Theophilus Akinfolaranmi FASHANU2, Ganiyu Ishola LAWAL1, Olusegun Olusoji SOREMEKUN1
1Department of Metallurgical and Materials Engineering,
2Department of Systems Engineering, University of Lagos, Nigeria
mohrabiat2000@yahoo.com, akinfolaranmi1@yahoo.com, gilawal@yahoo.com, oosoremekun@yahoo.com
Abstract
The analysis of the relative contribution of Zinc Oxide/Triethylene Amine mixes in controlling corrosion of mild steel in hydrogen cyanide environment is undertaken in this study.
In earlier works [1,2], the individual effects of some organic and inorganic inhibitors were studied using 20ml of three different concentrations (0.5M, 1.0M and 1.5M) of the inhibitors from which diethylene amine from the organic family and zinc oxide from the inorganic family were reported to be very effective. However, in this work, mixes of zinc oxide and triethylene amine in varying volumetric combinations were considered at 1M concentration.
The weight losses for the various mixes were very low ranging from 7.7mg to 44.0 mg, superior to the situation observed in [1] and [2]. The corrosion rates followed reducing pattern except for the solution containing 5ml zinc oxide which had a sudden rise from 0.0090 mmpy to 0.0484 mmpy after 168 hrs. The weight loss ratios were in fractions in the range 0.2857 to 0.8361.
It is concluded that the mixture of triethylene amine and zinc oxide is effective with increasing volume of amine rather than the individual inorganic or organic inhibitor specie.
Keywords
Collaborative influence, Inhibitors, Mild steel, Hydrogen cyanide environment
Introduction
The central point of the National Economic Empowerment and Development Strategy (NEEDS) of the Federal Government is the policy of increasing the contribution of non-oil sector particularly the Agricultural and Allied Products to the Gross Domestic Product of the country. The focus is adding value to the product with a view to enhancing the derivation of maximum benefit. For instance, a sustainable cultivation, harvesting and processing of cassava tuber is estimated to have the potential of yielding £4b for the economy [3]. However, the processing of cassava to extract the useful constituent poses its own problem.
Cassava is a tropical crop whose active compound is Hydrogen cyanide. Hydrogen cyanide in cassava root ranges between 5-4mg/100g of cassava roots, 100 - 400 mg/100 g in the leaves (Cooke, 1978) [4].
Processing of cassava tuber to extract the important unit often releases solution of hydrogen cyanide which progressively attacks the materials of construction of the processing equipment as a result of continuous intimate contact between the hydrogen cyanide solution so released and the material of construction. This scenario may lead to plant failure, due to hydrogen embrittlement which results likely in stress corrosion cracking, material wastage and to some extent food poisoning due to contamination of processed cassava with corrosion product. The implication of this is that the estimated £4b from cassava cultivation and processing may not materialized after all.
Works had been done [1] and [2] on the effects of some type of inhibitors on the behavior of mild steel in cassava fluid. The two different works revealed that a higher concentration of inhibitor has better corrosion reduction capacity than a lower concentration of inhibitor irrespective of the nature of the inhibitor(s). These works did not examine the influence of volumetric combinations of inhibitor mix on the corrosion behavior of mild steel in hydrogen cyanide. Thus, the intent of this paper was to examine the contribution of volumes of inhibitor in controlling corrosion of mild steel in Hydrogen Cyanide.
Methodology
Specimen procurement and preparation
Mild steel plate whose spectrometric compositional analysis is given in Table 1, obtained from a Metal Roofing Sheet Industry in Ikeja, Lagos, Nigeria, was used for the study. The steel plate was prepared into test size of 2.5 cm “ 5.0 cm. The specimen surface was machined and fine ground with emery paper in order of fineness. This was to prevent the setting up of local anodic/cathodic region which an uneven specimen surface would have created. The surface was subsequently pickled and degreased using mild acid and acetone solvent. The specimen was thereafter dried in an oven, weighed on a sensitive balance and placed in a desiccator to prevent corrosion prior to experimentation.
Table 1. Spectrometric compositional analysis of specimen
|
Elements |
C |
Mn |
Si |
Ni |
Cr |
P |
S |
Cu |
Sn |
|
% composition (Wt) |
0.0922 |
0.3022 |
0.0276 |
0.0194 |
0.0227 |
0.0101 |
0.0125 |
0.0717 |
0.0124 |
Corrodant and inhibitor mix preparation
Hydrogen cyanide fluid was extracted from freshly harvested cassava tuber from a local cassava processing yard. The pH of the extracted fluid was measured to be 6.1. The fluid was kept in a refrigerator to maintain its pH.
The Zinc Oxide inhibitor was commercially procured in solid form whilst the triethylene amine was procured in liquid form.
1M solution of Zinc Oxide was made and mixed with an equal concentration of triethylene amine in volumetric ratio 15/5, 10/10, 5/15 ZnO/(C2H5)3N to give volumetric combination of 20 ml for the various mixtures of the inhibitors.
Experimentation
The initial weight of the prepared 2.5 cm “ 5.0 cm specimen was taken before it was immersed in the cassava fluid containing different volumetric combination of Zinc Oxide (ZnO) and triethylene amine [(C2H5)3N]. Specimens were immersed in such a way that they were completely covered by the cassava/inhibitor mix solutions.
A ratio of 20 ml inhibitor mix to 200 ml corrodant was used for all the samples across the three volumetric combinations of inhibitors. Weighing was done every 72 hours for 480 hours. Specimens were removed, rinsed thoroughly in water and alcohol to remove the rust formed. Each specimen was subsequently weighed with a sensitive weighing balance to determine the weight loss.
The corrosion rate is evaluated using the formula proposed by Fontana [5]:
Corrosion rate (mm/y) = (87.6∙W)/(D∙A∙T) (1)
where W = Weight loss in milligrams, D = Density of specimen in g/cm3, A = Total surface area, T = Exposure time in hours.
The weight loss ratio which measures the efficiency of inhibition is expressed as:
W.L.R = Weight loss of specimen in inhibited solution /
Weight loss of specimen in uninhibited solution (2)
Table 2. Gravimetric Data of Corrosion Behaviour of Mild Steel in Cassava Fluid
Inhibited with 1M 20ml Triethylene Amine and 1M 20ml ZnO (adapted from [1] and [2])
|
1M 20ml Triethylene Amine |
|||||||
|
Time (Hrs) |
0 |
96 |
168 |
240 |
312 |
408 |
480 |
|
Initial weight (g) |
10.4535 |
- |
- |
- |
- |
- |
- |
|
Final weight (g) |
- |
10.44 |
10.429 |
10.425 |
10.421 |
10.417 |
10.416 |
|
Weight loss (mg) |
- |
3.9 |
24.4 |
29 |
32.3 |
36.3 |
38 |
|
C.R. (mm/y) |
- |
0.125 |
0.126 |
0.105 |
0.09 |
0.077 |
0.069 |
|
W.L.R. |
- |
1.716 |
0.992 |
0.901 |
0.895 |
0.85 |
0.795 |
|
1M 20ml ZnO |
|||||||
|
Time (Hrs) |
0 |
96 |
168 |
240 |
312 |
408 |
480 |
|
Initial weight (g) |
10.208 |
- |
- |
- |
- |
- |
- |
|
Final weight (g) |
- |
10.201 |
10.188 |
10.185 |
10.182 |
10.18 |
10.177 |
|
Weight loss (mg) |
- |
6.6 |
17.7 |
23.1 |
25.9 |
27.9 |
31.5 |
|
C.R. (mm/y) |
- |
0.06 |
0.101 |
0.083 |
0.072 |
0.059 |
0.057 |
|
W.L.R. |
- |
0.814 |
0.801 |
0.717 |
0.175 |
0.653 |
0.659 |
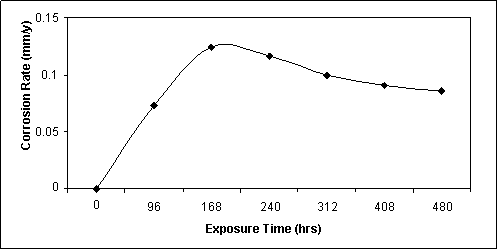
Figure 1. Corrosion rate against exposure time for specimen immersed in uninhibited cassava fluid
Table 4. Gravimetric Data of Corrosion Behavior of Mild Steel in Hydrogen Cyanide Inhibited with Zn0/(C2H5)3N Mixes
|
|
Value |
||||||||
|
Volumetric ratio [Zn0/(C2H5)3N] |
15/5 |
Time (Hrs) |
0 |
96 |
168 |
240 |
312 |
408 |
480 |
|
Initial weight (g) |
10.298 |
- |
- |
- |
- |
- |
- |
||
|
Final weight (g) |
- |
10.297 |
10.289 |
10.288 |
10.288 |
10.287 |
10.280 |
||
|
Weight loss (g) |
- |
1.000 |
9.400 |
10.800 |
10.100 |
10.800 |
17.700 |
||
|
C. R. (mmpy) |
- |
0.009 |
0.048 |
0.036 |
0.026 |
0.028 |
0.032 |
||
|
W.L.R |
- |
0.119 |
0.382 |
0.311 |
0.279 |
0.253 |
0.370 |
||
|
10/10 |
Initial weight (g) |
10.428 |
- |
- |
- |
- |
- |
- |
|
|
Final weight (g) |
- |
10.413 |
10.409 |
10.407 |
10.405 |
10.391 |
10.389 |
||
|
Weight loss (mg) |
- |
14.800 |
18.900 |
21.00 |
23.00 |
36.500 |
44.000 |
||
|
C. R. (mmpy) |
- |
0.133 |
0.097 |
0.057 |
0.064 |
0.076 |
0.079 |
||
|
W.L.R |
- |
1.827 |
0.768 |
0.652 |
0.637 |
0.855 |
0.921 |
||
|
5/15 |
Initial weight (g) |
10.232 |
- |
- |
- |
- |
- |
- |
|
|
Final weight (g) |
- |
10.218 |
10.209 |
10.204 |
10.202 |
10.197 |
10.193 |
||
|
Weight loss (mg) |
- |
13.700 |
22.200 |
27.800 |
29.800 |
34.800 |
38.500 |
||
|
C. R. (mmpy) |
- |
0.124 |
0.114 |
0.100 |
0.083 |
0.074 |
0.069 |
||
|
W.L.R. |
- |
1.6914 |
0.9024 |
0.863 |
0.825 |
0.815 |
0.805 |
||
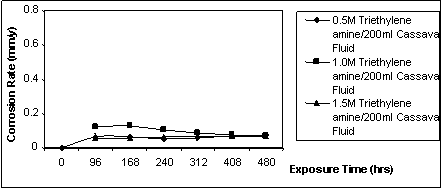
Figure 2. Corrosion rate against exposure time for specimen immersed in cassava fluid inhibited with triethylene amine [1]
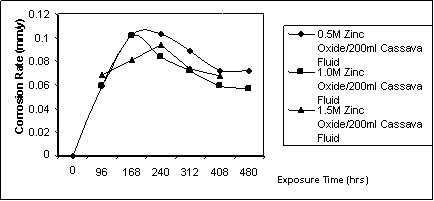
Figure 3. Corrosion rate against exposure time for specimen immersed in cassava fluid inhibited with zinc oxide [2]
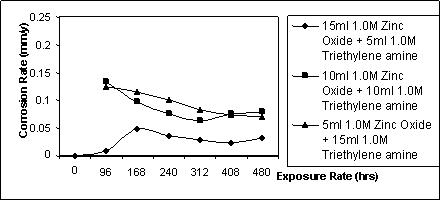
Figure 4. Cassava rate against exposure time for specimen immersed in cassava fluid inhibited with ZnO / (C2H5)3 mixes
![]()
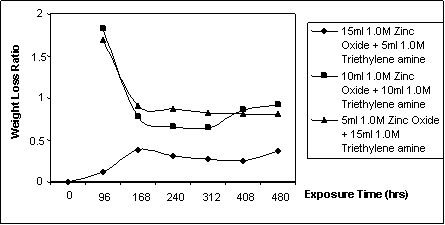
Figure 5. Weight loss ratio against exposure time for specimen immersed in cassava fluid inhibited with ZnO / (C2H5)3 mixes
Discussion of results
The obtained gravimetric data of corrosion behaviour of mild steel in cassava fluid inhibited with different volumetric mixes of zinc oxide and triethylene amine is presented in table 4 and better encapsulated in figures 4 and 5. Figures 2 and 3 were extracted from [1] and [2] respectively to give an apparent comparison of the individual effect of triethylene amine and zinc oxide with inhibitor mixture of zinc oxide and triethylene amine.
Figure 1 is the graph of the weight loss of specimen in uninhibited cassava solution. The figure indicated that the corrosion rate for this situation exhibited an accelerated increase in the early hours - 0.070 mmpy after 96 hrs, then 0.124 mmpy after 168 hrs and steadily reduces to 0.086 mmpy after 480 hrs. The total weight loss in the specimen in this situation is 47 mg over a period of 480 hrs.
Figure 2 is the observable pattern of the behaviour of mildsteel in cassava fluid in the presence of triethylene amine as inhibitor. The average weight loss is 38mg. The corrosion rate ranges between 0.057 - 0.125 mmpy. The behaviour of mild steel in cassava fluid inhibited with zinc oxide is displayed in Figure 3. The corrosion rate in this situation ranges between 0.057mmpy to 0.101mmpy while the total weight loss averages 35.65mg. Figure 4 is the graphical illustration of the corrosion rate of mild steel in cassava fluid inhibited with different volumetric mixes of zinc oxide and triethylene amine.
The total weight loss for the zinc oxide/triethylene amine mixes averages 25.85mg compared to 38mg in triethylene amine [1], 35.65mg in zinc oxide [2] and 47mg in uninhibited cassava fluid. These total weight losses for the volumetric mixes were apparently the lowest. It is equally apparent that there is a correlation between corrosion behaviour of mild steel in cassava fluid and the volumetric combination of the inhibitor mixes. 15/5 V/V zinc oxide/triethylene amine has the lowest corrosion rate. The corrosion rates were generally low compared to the earlier two works [1 & 2]. The rates followed reducing pattern except for the solution containing 5ml zinc oxide solution which had a sudden rise from 0.009mmpy to 0.048mmpy after 168 hours.
The efficiency of the inhibitors in reducing corrosion of mild steel in the cassava solution is measured by the weight loss ratio (equation 2.2). The weight loss ratio for the volumetric mixes of zinc oxide and triethylene amine displayed superior pattern to the behavior observed in [2]. That is, more lower than unity, thus, expressing the superior efficacy of the zinc oxide/triethylene amine mixes as inhibitor relative to a single zinc oxide inhibitor.
Conclusions
The work has revealed that corrosion rates of mild steel in hydrogen cyanide inhibited with mixture of zinc oxide/triethylene amine followed reducing pattern with a better weight loss profile compared to [1] and [2] across the three volumetric combinations considered. The best result, however, is achieved when the zinc oxide is of the higher volume (15/5) in the zinc oxide/triethylene amine mixes. The corrosion rate in this medium is extremely low.
The work showed that the admixture of zinc oxide and triethylene amine inhibitors exhibited a greater corrosion minimization compared to the situation of either of zinc oxide or triethylene amine alone.
It is therefore concluded that a better corrosion reduction capacity is achieved when inhibitors are applied in mixes rather than single.
Acknowledgement
The Management of Galvanizing Industries, Limited, Ikeja Lagos, Nigeria is acknowledged for providing the mild steel sheet. The assistance offered by the technical staff of the Department of Chemistry, University of Lagos, Lagos, Nigeria, in preparing the solution of the inhibitors is equally acknowledged.
References
[1] Amuda M. O. H., Lawal G. I., and Soromekun O. O., Inhibitive strength of some Organic Compounds on the Corrosion Behavior of Mild Steel in Cassava Fluid, 2005, an article sent for publication in The West Indian Journal of Engineering.
[2] Amuda M. O. H., Lawal G. I., Fashanu T. A., Soromekun O. O., Kamma C. M., Effects of Zinc Oxide and Sodium Sulphite Inhibitors on the Corrosion Behavior of Mild Steel in Cassava Fluid, 2006, Ife Journal of Technology (in press).
[3] Federal Ministry of Agriculture, Document on Government Policy on Accelerated Cassava Cultivation.
[4] Nartey F., Manihot Escalenta (Cassava) Cyanogenesis, Ultrastructive and Seed Germination, 1978, Munksgaard.
[5] Fontana M. G., Corrosion Engineering 3-rd ed., 1986, McGraw-Hill, New York.