Production of Detergent from Castor Oil
Abubakar Garba ISAH
Department of Chemical Engineering, Federal University of Technology, P.M.B. 65, Minna,
Nigeria, ag_isah@yahoo.com
Abstract
This research work was carried out with the objective of extraction of oil from castor seeds and its utilization to produce a synthetic detergent. Solvent extraction method was employed in extracting the oil and the total percent oil yield was found to be 23.8%. The experimentally determined saponification value of the oil was 183.7275mgKOH/g of oil. The detergent efficiency, determined as a measure of the foamability of the detergent was found to be 2.6cm. The pH tests revealed mildly basic properties. The color, scent and efficiency of the detergent were improved with the addition of bleaching agent, perfume and foaming agents respectively.
Keywords
Oil extraction, Castor seeds, Synthetic detergent
Introduction
The washing industry, usually known as soap industry, has roots over 2000 years in past, a soap factory having been found in the Pompeii excavation. However, among the many chemical process industries, none has experienced such a fundamental change in raw material as have the washing industries. Soap itself was never actually “discovered”, but instead gradually evolved from crude mixture of alkaline and fatty acid (George, 1984).
Scientifically, the term detergent covers both soap and synthetic detergent, or “syndets” but it is widely used to indicate synthetic cleaning compounds as distinguished from soap. Detergent differs from soap in their action in hard water. Although soaps are excellent cleansers, they do have disadvantages. As salts of week acids, they are converted by mineral acids into free fatty acids. These fatty acids are less soluble than the sodium or potassium salts and form precipitate or soap scum. Because of this, soaps are ineffective in acidic water. Also soaps form insoluble salts in hard water such as water containing magnesium, calcium or iron. The insoluble salts from bath rub rings, leave film that reduce hair luster, and gray/roughen textiles after repeated washings. Synthetic detergents, however, may be soluble in both acid and alkaline solutions and don’t form insoluble precipitate in hard water (www.chemistry.about.com/library/weekly/aa081301a.htm).
Many of the activities of man, starting from the primitive farming techniques to today’s high technology industrial activities have in small or large ways impacted negatively on man and his environment while the various products developed are highly desirable for the enhancement of the citizenry’s well being and sustenance of nations’ economy, the negative impacts precipitated by the introduction of its unwanted by-products into the ecological systems may be catastrophic if allowed to build up and uncontrolled.
Industrial revolution and evolution have been targeted principally at satisfying immediate changing demands rather than tailored towards a structured, wholesome and guided global program that will satisfy not only temporary human needs but are environmentally safe. The result of this is an increasing ecological degradation that has severely polluted water, land and air. (Odigure, 1998)
The detergent and soap making industries are no exceptions to the above trends, for while they provide us with cleansing agents, their processing and by-products are also a cause of public nuisance. For instance, detergents, unlike soaps, have proved very effective cleansing agents in hard and cool water whereas soap is often wholly ineffective under such condition. It was observed, however that many of these detergents were neither soluble nor biodegradable, that is they were so stable that when they flow into the soil in laundry sewage water, they remain unchanged, resisting conversion into less complex and more soluble substances. They thus, create suds and foams in fresh tap water, naturally occurring ground and surface waters.
To correct these odds require that environment issues be considered at the initial stages of conceptualization and development of synthetic detergents and other products as well. This will not only reduce pollution to the barest minimum but also save costs of treating these products. A deep knowledge and good manipulation of the process chemistry with a view to eliminating wastes is the most viable means of achieving this objective.
About 75 percent of detergents used in the early 1960s were for the most part made with alkyl benzene, made from propylene tetramer coupled to benzene. Tetra propylene is a very highly branched alkyl molecule. Research into the bacteria decomposition of alkyl based detergents showed that branched molecules are indigestible to the bacteria that must decompose the detergents when they reach the ground. To be biodegradable, a detergent should be based on straight chain alkyl molecule examples of straight chain alkyl molecules are the linear alkyl benzene sulphonates (LABS) and ricinoleic acid obtained from the castor bean. (Encarta online search)
This research project work is aimed at studying the replacement made to the non-biodegradable detergents. Production of environmentally friendly synthetic detergents (soft detergents) from castor oil has the dual advantage of using locally sourced raw material that can be grown and generating wastes that are appetizing to micro organisms (bacteria).
Materials and Methods
The castor seeds (or beans) were gotten from the Kabba local government area of Kogi state.
The operations involved in extraction of castor oil can be divided into two:
· Pre-treatment of the castor seeds
· Solvent extraction of the castor oil
Pre-treatment of castor seeds
The pre-treatment involves the preliminary preparations of the seeds in the following:
(a) Shelling: This involves the removal of the shells to obtain the seeds. It was done manually.
(b) Clearing: This involves the removal of foreign matter introduced during the sun drying and any unshelled seed.
(c) Drying: The moisture content of the seed was reduced using the electric oven. The oven was operated at 80oC for about three hours.
(d) Crushing: Mortar and pestle were used to reduce the sizes of the dry seeds so as to increase the interfacial area between the solvent and the seeds.
Extraction of castor oil
100ml of n-hexane was poured into the round bottom flask. 10g of castor beans was placed in the thimble and inserted in the center of the soxhlet extractor as seen in the figure. The extractor was heated at 70oC when the solvent was boiling; the vapor rises through the vertical tube into the condenser at the top. The liquid condensate drips into the filter paper thimble in the center which contains the solid sample to be extracted. The extract sips through the pores of the thimble and fills the siphon tube where it flows back down into the round bottom flask. This was allowed for 30mins after which the sample was removed from the tube, dried in the oven, cooled in the desiccators and weighed to determine the amount of oil extracted.
The experiment was repeated by placing 5g of the sample into the thimble again, and after every 30mins, the samples were withdrawn for drying and weighing. The miscella (extracted oil mixed with solvent) was heated at the end of the extraction to recover the solvent from the oil. The solvent free oil was then refined for further use.
Refining of castor oil
The refining was needed to remove gum from the extracted oil. Boiling water was added to the oil and the mixture stirred for 2 mins and allowed to stand in the separating funnel. The aqueous layer was then removed. The procedure was repeated to ensure removal of most gums. The de-gummed oil was collected and stored for use.
Determination of saponification value
2g of the oil was placed in a conical flask to which 25ml of ethanoic potassium hydroxide (0.1M) was added and the mixture allowed to boil gently for about 60 mins with shaking at regular intervals of 5mins.
Few drops of phenophthalin indicator, as specified by International Standards Organization (ISO 3657, 1988) was added to the warm solution and then titrated with 0.5M HCl. The end point was reached when the pink colour of the indicator just disappeared. The same procedure was followed for the blank.
The saponification value (sv) is given by:
sv = 56.1·N·(Vo – Vi)/m (1)
where: Vo = volume of HCl solution used for the blank test, Vi = volume of HCl solution for the determination, N = actual molarity of HCl used, and m = mass of sample
Production of detergent
0.1M sodium hydroxide solution was prepared by weighing 40g of NaOH Pellets into a beaker containing 100ml of water and shaking vigorously.
The electric hot plate was switched on; 30ml of castor oil in a stainless steel plate was placed on it and heated at 350C for about 2 mins. Caustic soda (0.1M) was added and the mixture stirred with a glass rod. 18M sulphuric acid was then added with constant stirring, and the reaction allowed to completion after which hydrogen peroxide (Bleaching agent) was introduced into the reaction mixture. When the foaming had subsided, the heating was continued to allow for more vaporization before putting off the heating system. Finally, perfume was added and the system was allowed to cool. The powdered detergent formed was the subjected to foamability test to ensure the effectiveness of the process.
The same steps were then followed, but this time palm kernel castor oil was used as the base material. The resulting powdered detergent formed was again collected and tested as above. The results are as shown in Tables 1 - 3, while the equations of the chemical reaction of the process are:
· Sulphation:
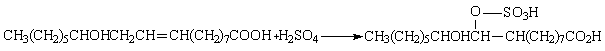
· Neutralization
![]()
· Vaporization
![]()
Foamability tests on detergents produced
About 2.0g of the palm kernel based detergent was added to a 500ml measuring cylinder containing 100ml of distilled water. The mixture was shaken vigorously so as to generate foams. After shaken for about 2mins, the cylinder was allowed to stand for about 10mins. The height of the foam in the solution was measured and recorded.
The same steps were followed using the detergent produced with the castor oil so that the foamability of the two could be compared. The results obtained are as recorded in the result section of this work.
Results
The results obtained for the percent volume of oil extracted, determination of saponification value and the production as well as the foamability tests of the detergent produced are as seen in Table 1-3.
Table 1. Average percent of oil extracted
|
Weight of raw sample W1(g) |
Weight of raffinate W2(g) |
Weight of oil extracted (g) |
Percent oil extracted W(g) |
|
25.00 |
16.45 |
8.55 |
34.20 |
|
20.00 |
13.20 |
6.80 |
34.00 |
|
30.00 |
22.00 |
8.00 |
26.67 |
|
35.00 |
26.80 |
8.20 |
23.43 |
|
40.00 |
29.10 |
10.90 |
27.25 |
Table 2. Comparing the saponification value of refined oil with the standard
|
Property |
Refined castor oil |
Standard value |
|
Saponification value |
183.7275mgKOH/g of oil. |
176 - 187 mg KOH/g of oil |
Table 3. Height of foam formed by the detergents produced
|
Detergent base material |
Height of foam formed in water (cm) |
|
|
Sample 1 (2.0g) |
Sample 2 (2.0g) |
|
|
Castor oil |
2.57 |
2.60 |
|
Palm kernel oil |
2.10 |
2.00 |
Discussions
The results obtained for the extraction showed an average percent oil extracted to be 29.11%. This value is low relative to similar works done by Isah, Alhassan and Garba, 2005 using n-hexane as solvnent with an average oil yield of 32.1%. The low yield could be attributed to the nature of the seeds and difference in solvent. The oil quality was very desirable as demonstrated from the saponification value of 183.7275 mg KOH/g of oil, which compares very favorably with that in literature (180.00 mg KOH/g of oil).
Moreover, the sulphation and neutralization reactions gave a powered detergent of high enough efficiently as seen from the result of the foamability tests. Usually, the efficiency of a washing powder is assessed through the amount of foam it is capable of producing. The presence of persistent foam exemplifies a good detergent (Bajar et al, 1995). The foam height of 2.6cm persisted for about 10 minutes and is higher than that formed by the palm kernel based detergent. The detergent formed was the result of the esterifications of the castor oil.
When ricinoleic acid (castor oil) is treated with concentrated H2SO4, its gives a complex mixture consisting of hydrogen sulphate (OSO3H) of ricinoleic acid in which the hydroxyl group is esterified and a compound in which the H2SO4 has added to the double bond. Esterification and addition do not occur together in the same molecule of ricinoleic.
The product which is known as Turkey red oil (sulphated castor oil) has good wetting properties. Neutralization of this with aqueous NaOH gave a detergent plus water. The reaction proceeded at temperatures between 35 - 40%. The water was vaporized by further heating and a solid (powdered) detergent was the result. The bleaching agent (H2O2) added helped to bleach the color of the castor oil so that milk colored detergent was produced. pH tests showed that the detergent exhibited basic property. The detergent can thus be described as amphoteric. This classification is characteristic of the intrinsic property of castor oil. This pH range is preferable to that of acidic as it is non - corrosive to the skin and cloths.
From the structure of castor oil (Ricinoleic acid) as shown under literature review, the sulphation reaction occurred at the hydroxyl group while the esterification reaction occurred at the ester linkages and this can be used to produce both soluble and insoluble soaps. Hence the detergent produced was the result of the esterification of the ricinoleic acid.
Conclusions
This research project have been undertaken to extract castor oil from its seed, refine this and use the same to produce a detergent.
The extraction was done using n-hexane as the solvent. The oil was refined by de-gumming to remove most gums. Sulphation and neutralization of the refined oil gave a detergent. Other operations like bleaching, perfuming and drying were done to improve color, scent and texture of the detergent. Although many other additives such as optical brighteners, extenders, re-deposition inhibitors, enzymes, etc. were not added, the active ingredients (surfactants) were used and as such the detergent efficiency was high.
The production of synthetics detergent from castor oil was successively done. The many desirable intrinsic qualities of castor oil makes it very useful in the detergent industry, thus castor oil can serve as a good substitute to petroleum and coal, the conventional detergent bases.
Recommendation
Literature reviews showed that castor oil has a linear structure and as such a detergent based on it should be digestible to soil micro organisms. It would therefore be a step in the right direction if this biodegradable property of the produced detergent is tested.
Acknowledgement
I acknowledge the contribution of Mr. Aduma Gideon to this work.
References
[1] Bajah S. T., Teibo B. O., Onwu G., Obikwere A., Senior Secondary Chemistry, Longman Publication, Lagos, Nigeria, pp. 153-156, 1995.
[2] George T. A., Shreve’s Chemical Process Industries, 5th ed. McGraw-Hill Book Company, New York, pp. 529, 1984.
[3] Isah A. G., Mohammed A., Garba M. U., Production, Refining and Evaluation of Castor Oil, Proceedings of 6th Annual Engineering Conference, School of Engineering Technology, Federal University of Technology, Minna, Nigeria, 15th - 17th June 2005, p. 50-56, 2005.
[4] Odigure J. O., Safety, Loss and Pollution Prevention in Chemical process Industries, Jodigs Associates Publication, Minna, Nigeria, pp. 89, 1998.
[5] How Does Soap Clean? [online]. ©2006 About. Available at: www.chemistry.about.com/library/weekly/aa081301a.htm.