Determination of Nitrite in Meat Products using a Metalloporphyrin Based Nitrite-Selective Membrane Electrode
Dana VLASCICI1, Elena Maria PICĂ2, Eugenia Făgădar COSMA3, Otilia BIZEREA1,
Viorica COSMA2
1 West University of Timişoara, 16 Pestalozzi St., Timişoara
2 Technical University of Cluj-Napoca, 15 C-tin Daicoviciu St., 400020, Cluj-Napoca
3 Institute of Chemistry, 24 M. Viteazul Ave., 300223, Timisoara, Romania
vlascici@yahoo.com, empica@yahoo.com, efagadar@yahoo.com, obizerea@yahoo.com, vioricacosma@yahoo.com
Abstract
The potentiometric response characteristics of a nitrite-selective electrode based on Co (III) tetraphenylporphyrins (TPP) in o-nitrophenyloctylether plasticized polyvinyl chloride membranes are compared. To establish the optimum composition of the membrane, different molar percents of cationic derivative (0-100 mol% relative to ionophore) were used. The influence of different plasticizers: o-nitrophenyloctylether, dioctylphtalate and tricresilphosphate on potentiometric answer were studied. Electrodes formulated with membranes containing 1 wt% ClCoTPP, 66 wt% o-NPOE, 33 wt% PVC (plasticizer:PVC = 2:1) and the lipophilic cationic derivative (10 mol%) are shown to exhibit high selectivity for nitrite over many anions, except the lipophilic anions perchlorate and thiocyanate. The electrodes based on Co (III) porphyrins were used for the potentiometric determination of nitrites in meat products. The results were compared with a colorimetric method used as the reference method. There was a good agreement between the potentiometric and colorimetric procedures.
Keywords
Metalloporphyrins, Cobalt (III) tetraphenylporphyrin, Nitrite, Anion-selective electrode, Meat
Introduction
The selective determination of many anions is difficult because the classical Hofmeister series is correlated with a preference for hydrophobic anions. This is the reason why highly hydrated anions such as fluoride, chloride and nitrite are difficult to monitor due to significant interference from more lipophilic anion species that may be present in the sample. So, in the field of anion-selective electrodes, the need for ionophores with improved selectivities and sensitivities is increased.
Metalloporphyrins used as ionophores in solvent/polymeric membrane electrodes can induce potentiometric anion selectivity patterns that differ significantly from the classical Hofmeister pattern. The ability of the porphyrins to have a Lewis acidic metal as the coordinating site, together with the fact that the binding affinity of this central metal could be controlled, to a large degree, by the surrounding porphin ring as well as the fifth or sixth ligand attached to the metal, makes them interesting anion carriers. Electrode selectivity towards anions in these cases is not governed by anion lipophilicity as in the case of dissociated ion-exchanger, but by specific chemical interactions between the metalloporphyrin from the membrane and the anions in the sample solution [1-5]. It has been shown that the nature of the ionophore-anion interaction is governed by the charge of the central metal ion within the porphyrin structure. The operative mechanism (neutral carrier versus charged carrier) must be known because the optimization of membrane permselectivity is highly dependent on the incorporation of additional membrane additives. It has been shown that electrode response and selectivity is enhanced by the addition of tetraphenylborate derivatives when the metalloporphyrins are acting as charged carriers, while tetraalkylammonium species are required as additives for membrane doped with ionophores that function as neutral carriers [6].
In the present paper, the potentiometric response characteristics of a nitrite-selective electrode based on Co(III)-tetraphenylporphyrin (ClCoTPP) in o-nitrophenyloctylether plasticized poly (vinyl chloride) membranes are compared. According to the previously published procedures [7] we have synthesized and purified chloro-(5,10,15,20-tetraphenylporphyrinato)-cobalt(III) (ClCoIIITPP). The product was characterized by UV-VIS, IR and 1H-RMN spectra, elemental analysis and cyclic voltametry [8] and used as ionophore in liquid membrane ion-selective electrodes.
A lipophilic cationic derivative is required to optimize potentiometric selectivity for nitrite over other anions. To establish the optimum composition of the membrane, different molar procents of cationic derivative (0-100 mol% relative to ionophore) were used. Also, the influence of different plasticizers like o-nitrophenyloctylether, dioctylphtalate and tricresilphosphate on potentiometric answer was studied. Electrodes formulated with membranes containing 1 wt% ClCoTPP, 66 wt% o-NPOE, 33 wt% PVC (plasticizer:PVC = 2:1) and the lipophilic cationic derivative (10 mol%) are shown to exhibit high selectivity for nitrite over many anions, except the lipophilic anions perchlorate and thiocyanate. The electrodes having the optimum composition of the membrane presents reversible and near-Nernstian response toward nitrite in a concentration range of 10-1 - 5∙10-5 M with a good stability in time. The electrode was completely characterised: slope, selectivity, detection limit, response time, lifetime and working pH range were determined.
The high degree of nitrite selectivity exhibited by the electrodes based on Co(III) porphyrins makes them potentially useful for the analytical control of nitrites in meat products. For the potentiometric determination of nitrite in four types of meat products, measurements were carried out by multiple standard additions method. The obtained results were compared with a colorimetric method used as the reference method. Both of the methods were simultaneously applied to the extracts of meat products. There was a good agreement between the potentiometric and colorimetric procedures.
Experimental
Reagents
Chloro-(5,10,15,20-tetraphenylporphyrinato)-cobalt(III) (ClCoIIITPP) was synthesized in accordance with previously published procedures and characterized by UV-VIS, IR, 1H-RMN spectra, cyclic voltametry and elemental analysis. For polymer membrane preparation, o-nitrophenyloctylether (o-NPOE), dioctylphtalate (DOP), tricresil-phosphate (TCP) trioctylmethyl-ammonium chloride (TOMACI), polyvinyl chloride (PVC, high mol. wt.), tetrahydrofuran (THF, distilled prior to use) were purchased from Fluka and Merck.
All aqueous solutions were prepared with salts of the highest purity available. The sample solutions for all potentiometric measurements consisted of sodium salts of the given anions in 0.05 M 4-morpholino-ethanesulfonic acid (MES), adjusted to pH = 5.5 with NaOH.
ISE membrane formulation and EMF measurements
Membranes employed to construct ion-selective electrodes consisted of: 1 wt% ClCoTPP, 66 wt% o-NPOE (DOP, TCP), 33 wt% PVC and TOMACl (as cationic additive in membrane) 0-50 mol% relative to ionophore. First, the ionophore and the additive were dissolved in the solvent mediator. Then the PVC and sufficient amount of THF were added and mixed to obtain a transparent solution.
This mixture was transferred onto a glass plate of 20 cm2, and the THF was allowed to evaporate at room temperature leaving a though flexible membrane trapped in a PVC matrix.
The cast membranes were approximately 150 μm thick. Potentiometric measurements were performed with Hg/Hg2Cl2/bridge electrolyte / sample / ion-selective membrane / Ag(Hg) / internal cable galvanic cell.
Hg/Hg2Cl2/bridge electrolyte/sample/ion-selective membrane/Ag(Hg)/internal cable
The bridge electrolyte consisted of 0.1 M KNO3. Prior to EMF measurements, the electrodes were conditioned for 24 h in a 0,1 M NaCl/0,01 M NaNO2 solution.
All experiments were performed at ambient temperature (22±2 °C). Potentials were measured using a Hanna Instruments HI8817 pH/mV-meter. Potentiometric selectivity coefficients were determined according to the separate solution method [9] by using the experimental EMF values obtained for 0.1 M solutions of the test anions and a theoretical slope of -59.2 mV /pNO2 for the primary anion. Activity coefficients were assumed to be constant for all analyte anions, and no correction was made for the slight changes in the liquid junction potential of the reference electrode.
HCl and NaOH solutions having different concentrations were used to study the pH dependence of nitrite-selective electrode. The pH of the test solutions was measured using a glass electrode. The effect of the pH of the nitrite solutions on the measured potential was achieved by using solutions of NaNO2 from 10-1 to 10-5 M in MES buffer having different values of pH: 4.5, 5.5, and 6.5.
Results and discussion
The potentiometric response characteristics of the liquid/polymeric membrane electrodes based on Co (III)[TPP]Cl were determined using solutions from 10-5 to 10-1 M of the following anions: ClO4-, SCN-, NO2-, F-, Salicylate-, NO3-, Cl-, Br-. To establish the optimum composition of the membrane, 4 electrodes were prepared having the following composition: 1 wt% ClCoTPP, 66 wt% o-NPOE, 33 wt% PVC and various concentrations of cationic additive: 0 mol% (E1), 10 mol% (E2), 50 mol% (E3) and 100 mol% (E4) TOMACl (relative to the ionophore). The selectivity coefficients of these 4 electrodes are comparatively presented in figure 1 and their potentiometric responses to nitrite in MES buffer 5.5 are presented in figure 2.
![]()
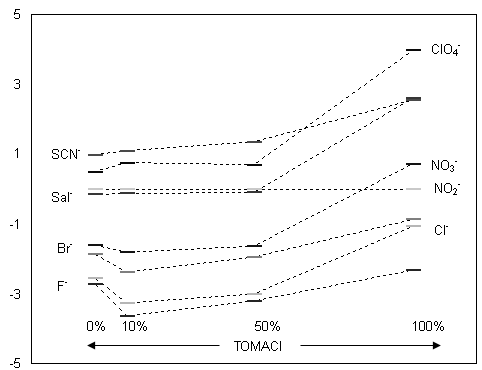
Figure 1. logK selectivity coefficients for PVC/o-NPOE membranes doped with ClCoTPP and various concentrations of cationic additive
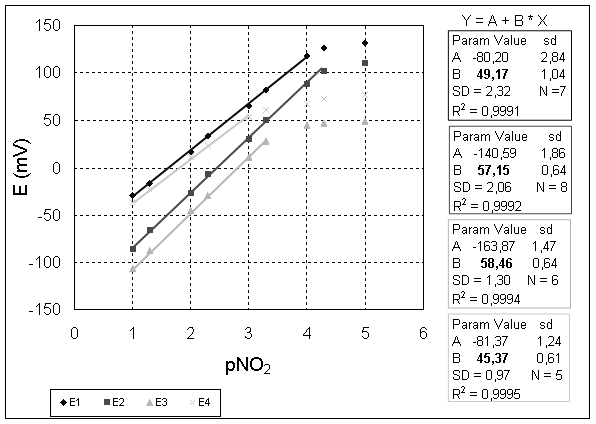
Figure 2. Potentiometric responses of membranes containing ClCoTPP in PVC/o-NPOE matrix to nitrite in MES buffer, pH=5.5 and various concentrations of cationic additive
In figures 1 and 2, it can be seen that:
· The lipophilic cationic derivative (in our case TOMACl) improves the potentiometric characteristics (selectivity and sensitivity) of the electrodes;
· The electrodes E2 and E3 have an anti-Hofmeister selectivity pattern. They exhibit preference for nitrite ions over all the anions tested, except the highly lipophilic perchlorate and thiocyanate.
· The electrode with an increased quantity of cationic additive (E4) has a Hofmeister selectivity pattern; nitrate and salicyate anions interfere besides perchlorate and thiocyanate. Also, a drastic decrease of slope and linear range of the electrode can be observed.
· The widest linear range, the lowest limit of detection, the best values of the selectivity coefficients and a near-Nernstian slope were observed for the electrodes with 10 mol% of cationic additive (TOMACl) added. So, we can conclude that the optimal composition of a nitrite-selective electrode membrane is: 1 wt% ClCoTPP, 66 wt% o-NPOE, 33 wt% PVC (plasticizer: PVC = 2:1) and 10 mol% of TOMACl (relative to ionophore).
For the electrodes having the optimum composition of the membrane, we have studied the influence of different plasticizers on potentiometric answer. Three electrodes with o-NPOE, DOP and TCP as plasticizers were comparatively studied. The obtained results are given in table 1 and plotted in figure 3.
Table 1. Selectivity coefficients toward nitrite for membrane electrodes containing different plasticizers
|
Plasticizer |
|
|||||
|
NO2- |
ClO4- |
SCN- |
Cl- |
Sal- |
NO3- |
|
|
o-NPOE |
0.0 |
+0.64 |
+1.04 |
-3.41 |
-0.18 |
-2.03 |
|
DOP |
0.0 |
+0.68 |
+0.77 |
-2.47 |
-0.28 |
-1.84 |
|
TCP |
0.0 |
+0.95 |
+0.72 |
-2.86 |
-0.29 |
-2.00 |
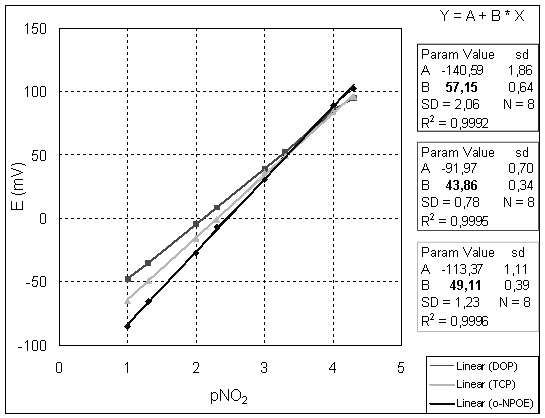
Figure 3. Potentiometric responses to nitrite of electrodes with membranes containing Co(III)[TPP]Cl and different plasticizers
From the above-presented results, it can be seen that the selectivity pattern was essentially the same for all three compositions of the membrane. Near-Nernstian slope is obtained for electrodes plasticized with o-NPOE but in the case of DOP and TCP, the slopes were below the Nernstian value. It results that o-NPOE is better than other plasticizers in ClCoTPP based ion-selective electrodes and the optimal composition of the membrane is the above presented one.
For the electrodes having the optimum composition of the membrane, the response time, detection limit, lifetime and working pH range were determined. The average time for the nitrite-selective electrode to reach 95% of the final potential value after successive immersion of the electrode in a series of nitrite ion solutions, each having a 10-fold difference in concentration, was measured. The obtained response time was about 60 s.
The evolutions of the nitrite-selective electrode slopes in time, measured for three electrodes having the optimum composition of the membrane, are presented in figure 4. There was a little decrease of the slope in time, but it still remains, after a month of intensive use, in the analytical useful range.
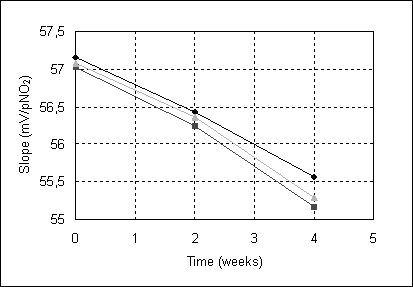
Figure 4. Evolution of slopes in time
The pH dependence of ClCoTPP based electrodes, having the optimal composition of the membrane, is presented in figures 5 and 6.

Figure 5. The pH dependence of CoTPP-based electrode
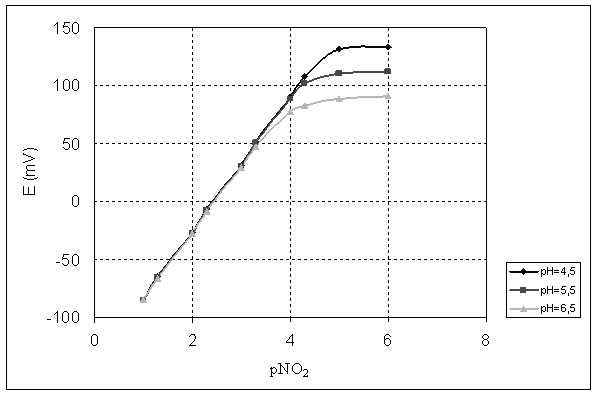
Figure 6. The effect of sample pH on the potentiometric response to nitrite
In figures 5 and 6 it can be seen that the detection limit of the electrode strongly depends on the pH sample. Literature data [10] provides that there are two different mechanisms, which govern the pH dependence of the metalloporphyrin-based electrodes. At high pH values, the hydroxyl concentration is increased and the potentiometric answer of the electrode can deviate from linearity. In acid media, where the hydroxyl concentration is low, the electrode responded probably to H+ ions because the membranes contained neutral carriers of amine nature. This is the reason why, the useful pH range of these electrodes is between 4.0 and 5.5.
Analytical Applications
Nitrites are widely used in the production and preservation of cured meat products, but are well known as toxic substances. The major effect of nitrites is the induction of methaemoglobinemia as a result of nitrite reduction with hemoglobin. They can also react with some amines or amides present in the stomach forming N-nitroso compounds with carcinogenic action. These are the reasons why, the nitrite content in food industry must be controlled and their determination must be currently done for checking the quality of meat products.
In the present work, the nitrite-selective electrode metalloporphyrin based was used for the analytical determination of nitrite content from four types of meat products. Samples of salami, corned beef, sausage and pate were taken and the extraction method recommended by legislation was applied.
The above-mentioned results indicate that the interfering anions to nitrite are thiocyanate and perchlorate, both missing from the meat samples. The main problem for the potentiometric determination of nitrites in meat products is the presence of chloride at high concentrations but adding a silver sulphate solution to the extracted samples solved it [11].
The nitrite content from each sample was spectrophotometric determined. A low concentration of nitrite was found, so, the samples were spiked with nitrite quantities of 100 mg/kg. For the potentiometric determination of nitrite, measurements were carried out by multiple standard additions method. The obtained results were compared with a colorimetric method used as the reference method. Both of the methods were simultaneously applied to the extracts of meat products. The obtained results are comparatively presented in table 2.
Table 2. Comparative results of the nitrite from meat products determination through potentiometry and spectrophotometry
|
Meat product |
Nitrite added (mg/kg) |
Nitrite determination |
RE (%)b |
|||
|
Potentiometric method |
RSDa (%) |
Spectrophotometric method |
RSD (%) |
|||
|
Pate |
100 |
119.4 ± 0.5 |
0.4 |
119.0 ± 0.4 |
0.3 |
+0.3 |
|
Corned beef |
100 |
188.9 ± 1.7 |
0.9 |
188.2 ± 0.6 |
0.3 |
+0.4 |
|
Salami |
100 |
162.5 ± 1.6 |
1.0 |
161.5 ± 0.7 |
0.4 |
+0.6 |
|
Sausage |
100 |
126.4 ± 0.9 |
0.7 |
125.8 ± 0.4 |
0.3 |
+0.5 |
a Relative standard deviation
b Relative error of the potentiometric vs. spectrophotometric methods
There was a good agreement between the potentiometric and colorimetric procedures showing that the nitrite-selective electrode based on cobalt (III) tetraphenylporphyrin chloride is analytically useful for the determination of nitrites in food products.
Conclusions
The results of the present study show that electrodes having cobalt (III) tetraphenylporphyrin chloride as ionophore in o-nitrophenyloctylether plasticized polyvinyl chloride membranes, realized with an internal electric solid contact, exhibit a relatively high selectivity for nitrite over many anions. The most important characteristics of the electrodes having the optimum composition of the membrane are: slope: - (57.1 ± 1.1) mV/decade; linear range: 10-1 - 5∙10-5 M; detection limit: 4∙10-5 M; response time: 60 s.
The electrode was successfully tested for determination of nitrite in meat products.
Acknowledgements
This work was supported by the Romanian Ministry of Education and Research, under the National Program PNCDI - INDAL 5451/249/ 2004.
References
[1] Steinle E.D., Schaller U., Meyerhoff M.E., Response characteristics of anionselective polymer membrane electrodes based on gallium(III), indium(III) and thallium(III) porphyrins, Anal. Sci., 14(1), 1998, p. 79-84.
[2] Malinowska E., Niedziolka J., Meyerhoff M.E., Potentiometric and spectroscopic characterization of anion selective electrodes based on metal(III) porphyrin ionophores in polyurethane membranes, Anal. Chim. Acta, 432, 2001, p. 67-78.
[3] Khorasani J.H., Amini M.K., Motaghi H., Tangestaninejad S., Moghadam M., Manganese porphyrin derivatives as ionophores for thiocyanate-selective electrodes: the influence of porphyrin substituents and additives on the response properties, Sensors Actuators B, 87(3), 2002, p. 448-456.
[4] Malinowska E., Meyerhoff M.E., Role of axial ligation on potentiometric response of Co(III) tetraphenylporphyrin-doped polymeric membranes to nitrite ions, Anal. Chim. Acta, 300, 1995, p. 33-43.
[5] Chaniotakis N.A., Park S.B., Meyerhoff M.E., Salicylate-selective membrane electrode based on tin (IV) tetraphenylporphyri, Anal. Chem., 61, 1989, p. 566-570.
[6] Bakker E., Malinowska E., Schiller R.D., Meyerhoff M.E., Anion-selective membrane electrodes based on metalloporphyrins: The influence of lipophilic anionic and cationic sites on potentiometric selectivity, Talanta, 41(6), 1994, p. 881-890.
[7] Adler A., Longo F.R., Goldmacher J., Assour J., Korsakoff L., A simplified synthesis for mesotetraphenylporphirine, J. Org. Chem., 32, 1987, p. 476-482.
[8] Făgădar-Cosma E., Vlascici D., Făgădar-Cosma Gh., Bizerea O., Chiriac A., Study of electrochemical behavior of metal-porfines with Co(II) and Co(III). Selective nitrite electrode based on Cl-(5,10,15,20-tetraphenyl-21H 23H-porphyrinate-N21,N22,N23,N24)-Co(III), Revista de Chimie (Bucharest), 55(5), 2004, p. 872-850.
[9] Guilbault G.G. & others, Recommendations for nomenclature of ion-selective electrodes, Pure Appl. Chem., 48, 1976, p. 127-132.
[10] Egorov V.V., Rakhman’ko E.M., Rat’ko A.A., Metalloporphyrin-based anion-selective electrodes with unusual selectivity, J. Anal. Chem., 57(1), 2002, p. 46-53.
[11] Perez-Olmos R., Yoldi I., Ruiz M.P., Merino J.M., Potentiometric Determination of Nitrite in Meat Products Using Nitrite-Selective Electrode, Anal.Sci., 14(5), 1998, p. 1001-3.