Design of an Alkaline Fuel Cell
Mohammed ALHASSAN, Mohammed UMAR GARBA
Federal University of Technology Minna, mohakusi2003@yahoo.com, tanimugarba@yahoo.com
Abstract
This paper present the design of an alkaline fuel cell operating between
100-250oC. Hydrogen was bubbled into one arm while oxygen into the
other. A meter was connected between the electrodes which detects the current
flowing through. From the analysis of the results, the input and output of the
components were 170kh/hr, the enthalpy of hydrogen and oxygen into the cell are
288.98kJ/hr and 147.06kJ/hr while the enthalpy of potassium carbonate and water
leaving the cell are - 1.11·106 kJ/hr and - 9.71 x 105
kJ/hr respectively. Other results are the volume of the cell 303.3/0.30 l/m3,
power generated 121W and cost of the fuel cell N 158371.04k.
Keywords
Alkaline, Fuel cell, Enthalpy
Introduction
In 1893, the English physicist William R. Grove, working from the knowledge that winning an electric current through water would produce hydrogen and oxygen showed that combining hydrogen and oxygen could produce water and an electric current. Grove’s demonstration opened the way to a new electrical power source: the fuel cell, but little was done with the concept for well over a century. Fuel cells were used to provide electrical power to the Apollo Moon capsule and other spacecraft, but failed to reach a wilder market. They are now undergoing rapid development.
The use of fuel cells in both stationary and mobile power applications can offer significant advantages for the sustainable energy conversion. Benefits arising from the use of fuel cells include efficiency and variability, as well as economy, unique operating conditions, planning flexibility and future development potential. By integrating the application of fuel cells, in series with renewable energy storage and production methods, sustainable energy requirement may be realized (Melean et al, 2001).
The general design of most fuel cells is similar except for the electrolyte. Several different substances have been used is the electrolyte in fuel cells, each with their own advantages and disadvantages. The five main types of fuel cells as defined by their electrolyte are: alkaline fuel cells (AFCs); solid polymer fuel cells (also known as proton exchange membrane fuel cells or PEMs); phosphoric acid fuel cells (PAFs); molten carbonate fuel cells; and solid oxide fuel cell. (SOFCs);.alkaline and solid polymer fuel cells operate at a lower temperatures (50 - 260°C) and are mainly designed for transportation application. The other three operate at higher temperatures (up to 1000°C for solid oxide fuel cell) and are being developed for use in co - generation and large central power plants. PAFs are being used in the Department of Defence (DOD) to fuel all demonstration program (Franklin (2004)).
Alkaline Fuel Cells (AFCs)
This is one of the oldest designs and the first practical fuel cells to be developed for powering electrical systems on space craft. It exploits the high conductivity and boiling point of a concentrated alkaline solution (potassium hydroxide) and runs at 100 - 250°C. Nickel and silver electrodes are used. The alkaline electrolyte means that carbon dioxide, which degrade (carbonate) the electrolyte must be completely eliminated only highly purified hydrogen and oxygen can be used, the cost of which imposes a severe limitation to applications other than space (Noburu Itoh, 1990).
In general form, a fuel cell consists of a porous anode and a porous cathode, with these two electrodes separated by a electrolyte. An oxidant is fed to the cathode to supply hydrogen while a fuel is fed in the anode to supply hydrogen. The electrolyte supports the transfer of ions between anode and cathode to support the reverse electrolysis reaction.
The fuel processor or reformer extracts hydrogen- rich gas from natural gas or other fuel, amity carbon dioxide and trace amounts of carbon monoxide. There are three common methods of processing fuels to create the hydrogen required by the fuel cells. Steam reforming is a simple process involving the reaction of light hydrocarbon fuels with steam. Partial oxidation is the incomplete burning of a fuel and is used to process heavier hydrocarbon liquids. The third reforming methods, gasification causes coal to be “gasified” by reacting coal with oxygen and steam at high temperatures.
Firstly, the power conditioner section of a fuel cell power plant most often consists of an inverter, which converts the DC electricity to AC electricity. The power conditioner can also regulate the voltage and current output from the fuel cells to accommodate variations in load requirements.
Basics of the Design of Fuel Cell
Currently, Dow, Dupont, Etek and others produced their own electrolytes using different mixtures in order to develop a membrane which resists electrons, but encourages the travel of hydrogen ions. Dupont (which produces nafion) was the first to produce the membrane, but recently, Dow and others have improved on the design which provides a more powerful cell. When designing, these electrolytes must never come in contact with copper as they will easily be poisoned and loose their conductivity. If copper could be used as current collectors, or for electrode substrate materials, an appreciable increase in current would result because of the lower Ohmic resistance (Lee W. L., 1998).
The objective of this design is to generate electricity from fuel cell in as much as possible because of its various application in real life and it efficiency and minimize total dependence on hydroelectricity.
Materials and method
Material Balance over Fuel Cell
The input to the cell initially is hydrogen and oxygen.
2H2 + O2 → 2H2O (1)
The water produced from equation 1 combined with KOH and CO2 to produce K2CO3 and more water.
2 KOH + CO2 → K2CO3 + H2O (2)
2 KOH + CO2 +H2O → K2CO3 + 2H2O (3)
The Mass entering or leaving the cell is thus
C = n·mw (4)
The total mass entering or leaving the cell is:
CT = nT·mw (5)
From Faraday’s law of electrolysis:
M = Z·I·T ` (6)
and
Q = I·t (7)
Where Q is the quantity of electricity which flows and Z is the electrochemical equivalence of the substance. The electrochemical equivalence of platinum is Z = 0.5 kg/Coul and M is the mass of water which is equal to M = CT therefore M is 72kg.
Therefore, the current following for one (1) hour is obtained from equation 7 and voltage from equation 8.
Power = I·V (8)
Energy Balance over Fuel
The Energy balances across the fuel cell is given as:
DH = M· (Cp·DT + Hf) (9)
where M is the mass of components in the fuel cell, CP is the heat capacity of the components which is given as:
Cp = a + b·T = c·T2 + d·T3 (10)
DT is the temperature change between the temperature inside the cell and the reference temperature, Hf is the enthalpy of formation (Himmelblau, 1996), and Hf = Hf/mw.
The energy input for hydrogen and oxygen and output for K2CO3 and H20 were obtained from equation (9).
Specification of Fuel Cell
Given the densities of the components in the fuel cell, the volume of each component is given as:
Vcomp = m/r (11)
where m is the mass of the components, and r is the density of each of the component.
The total volume is thus:
Vcell = ĺVcomp (12)
Vcell = 0.30 m3
But VCell = l · b · h. Assuming that h = 1/2 l and b = 1/3 l
VCell = l · 1/2 l · 1/3 l
Therefore
l = 1.8 m, h = 0.9 m and b = 0.6 m
l = x1 + x2 + x3
x1 and x2 are the height of the electrodes respectively while x3 is the length between the electrodes.
x1 = x2 = 0.15
x3 = 1.2 m
The dept of the electrode in the fuel cell is he = 0.675 m
Material Specifications
The Fuel cell is constructed with polyvinyl chloride (PVC) and the two electrodes are made up of nickel.
Costing of Fuel Cell
The cost of fuel is given as:
Ce = C, Sn (13)
where Ce = purchased equipment cost, S = Characteristic size parameter, C = Cost Constant, and n = index for that type of equipment
From the specification of the equipment the volume was found to be Vcell = 0.30 m3
S = Vcell/m3 S = 0.30 (14)
C = 2400
n = 0.60
So, the cost of the cell is therefore Ce cell = 158.
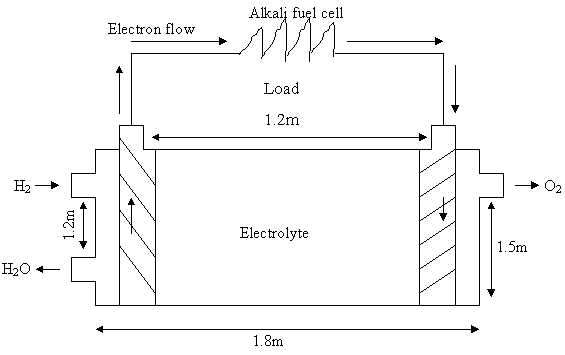
Figure 1. Alkali fuel cell
Results and Discussions
The results of the material and energy balances over a fuel cell are shown in Table 1, 2 and 3.
Table 1. Shows the results from the material balances over fuel cell
|
Components |
Cin (Kg) |
Cout (Kg) |
|
H2 |
4.00 |
0.00 |
|
O2 |
32.00 |
0.00 |
|
KOH |
72.00 |
0.00 |
|
CO2 |
44.00 |
0.00 |
|
K2CO3 |
00.00 |
98.00 |
|
H2O |
18.00 |
72.00 |
|
TOTAL |
170.00 |
170.00 |
Table 2. Shows the result from energy balance over fuel cell
|
Components |
DH in (kg/hr) |
DH out (kg/hr) |
|
H2 |
288.98 |
0.00 |
|
O2 |
147.06 |
0.00 |
|
KOH |
0.00 |
0.00 |
|
CO2 |
0.00 |
0.00 |
|
K2CO3 |
0.00 |
-1.11 x 106 |
|
H2O |
0.00 |
-9.71 x 105 |
|
Total |
436.04 |
-207 x 106 |
Table 3. Shows the result of some important parameters
|
Parameters |
Values |
|
Volume of Cells (l/m3) |
303.31/0.30 |
|
Length of Cell (m) |
1.8 |
|
Height of Cell (m) |
0.9 |
|
Base of Cell (m) |
0.6 |
|
Height of the electrode (m) |
0.25 |
|
Depth of the electrodes (m) |
0.675 |
|
Heat load (KJ/hr) |
2.07 · 106 |
|
Power (W) |
121 |
|
Voltage (V) |
3.028 · 103 |
|
Cost of Cell ( |
158371.04 |
In table 1, the total mass input and output of the components were 170 kg, this provided the information needed for the sizing of the cell.
It is observed from the energy balance in Table 2, that the enthalpy of hydrogen into the cell was 288.98 KJ/hr and 147.06 KJ/hr while the enthalpy of potassium carbonate and water leaving the cell were -1.11·106 KJ/hr and -9.71·105 KJ/hr respectively. This shows that the reaction is an exothermic one and therefore the materials for the construction should be carefully selected if the heat produced is not needed but if the needed means of removing the heat should be employed.
In table 3, it shows that the fuel cell generated power of 121W and a volume
of 303.31l/0.30 l/m3. The cost obtained for the fuel cell was N
158371.04k which is quite acceptable and affordable for producing the equipment
for small and medium scale enterprises (SMEs).
Conclusion
The alkaline fuel cell was designed to operate between 100-250°C. The total mass was 1709 kg and the output of the enthalpy was in excess due to exorthermic nature of the cell. The powere generated, the volume of the cell are 121W and 303.311/0.30 l/m3 respectively. The cost obtained for the cell was 158371.04 k which is quite acceptable and affordable for SMEs.
References
[1] Malean et al, An Assessment of Alkaline Fuel Cell, International Journal of Hydrogen Energy, John Wiley and Son, Vol 1and 2, 2001, p. 2-3.
[2] Noburu Itoh, New Trick for Old Power Source, IEEE, Spectrum 40, 1990, p. 43.
[3] Lee W., Lizinger T. A. et al, Effect of Platinum Catalyst on propane Oxidation at Elevated Pressure, Technical Report, 1998, p. 1 & 4.
[4] Fuel Cell 2000, Fuel Cell Basics, Application, Edited 2004, Retrieved 4/07/05, 2005, Available at: http://www.fuelcell.org.
[5] Grove William R., On a Gas Voltaic Battery, Proceeding of the Royal Society, 1843, p. 268 - 278 & p. 346 - 354.
[6] Franklin H., How a Fuel Cell Operates, Construction Engineering Research Laboratories, 2004, p. 7.