Corrosion and Stress Corrosion Behaviors of Low and Medium Carbon Steels in Agro-Fluid Media
Ayo Samuel AFOLABI
Abstract
Investigations were carried out to study critically the corrosion behaviour and Stress Corrosion Cracking (SCC) of low and medium carbon steels in cassava and cocoa extracts by weight loss measurement and constant extension to fracture method respectively. The results obtained showed that medium carbon steel is more susceptible to corrosion than low carbon steel in both media. SCC is also more in medium carbon steel than low carbon steel in the two media under study. These deductions are due to higher carbon content in medium carbon steel coupled with various aggressive corrosion constituents contained in these media. Hydrogen embrittlement, as well as carbon cracking, is responsible for SCC of these materials in the agro-fluid media.
Keywords
Corrosion, SCC. LCS, MCS, Cocoa extract, Cassava extract
Introduction
Corrosion has always been regarded as
costly problem to mitigate in any manufacturing, production and processing
industries because of its pervasive nature. The best approach is to look at the
corrosion behaviour of the metals being used in these
industries so that an approximate method of prevention could be ascertained.
Low and medium carbon steels are known for their versatility in many of these
processing industries.
Stress corrosion cracking (SCC) is the cracking indicated from the combined influence of tensile stress and a corrosive environment (Saxena et al, 2006). The impact of stress corrosion cracking on a material usually falls between dry cracking and the fatigue threshold of the material. The required tensile stresses may be in the form of directly applied stress or in the form of residual stress. Various findings have also been reported on other variables affecting SCC of metallic materials in many media [Daret et al, (1995), Lumsden et al, (1995), Miglin et al, (1995), Casales et al, (2002), Raman and Muddle, (2004) and Rogante et al, (2006)].
Several research efforts have been
reported about the anti-oxidation properties of cocoa products [Ziegleder and Sandmeir, (1982), Basaga et al, (1997), Azizah
et al, (1999), Arts et al, (1999) and Osman
et al, (2004)] while Moerck and Ball (1974)
and Dawson and Gartner (1983) opined that metallic materials could actually be
susceptible to oxidation in aerated cocoa extract as a result of extensive
stress and compositional nature of the material. Cassava on the other hand,
contains large amounts of cyanogenic glucosides (Oboh and Akindahunsi, 2003), tannic acid (Hahn, 1992), lotaustrlin (
The objectives of this work are to investigate critically the stress corrosion behaviour of low and medium carbon steels in cassava and cocoa extracts, study the controlling factors during SCC and compare the corrosion behaviour of these carbon steels in the agro fluids.
Materials and Methods
The materials used in this study were 8mm diameter low and 12mm diameter medium carbon steels. Their chemical compositions as supplied by the manufacturer are shown in Table 1. Eight tensile specimens each were prepared from these materials using lathe machine as shown in Figure 1.These samples were austenized at 900oC for 30 minutes, then quenched in water and tempered at 350oC for one hour. Each samples were separately immersed in fresh cocoa and cassava extracts which acted as corrosion media as shown in Figure 2. Each of the immersed tensile specimens in the corrosion media was removed at every four days interval and pulled in tension to fracture using tensometer. The percentage elongation and reduction in cross-sectional area, and stress-strain graph were obtained for each tensile specimen. The time to fracture for each of the tensile specimen was also measured with the aid of a stopwatch. [Sriram and Tromans, (1985), Le and Ghali, (1993), Rondelli et al,, (1997) and SinghRaman et al, (2004)]. Weigth loss measurement was conducted for both samples in the two agro--fluids at every four days interval using the procedures and precautions described by Che et al, (2005), Ashassi-Sorkhabi et al, (2006) and Jabeera et al, (2006).
Table 1. Chemical Composition of Steels Samples
Steel Sample |
% Composition |
||||||
|
C |
Si |
Mn |
P |
S |
Cu |
Fe |
|
|
Low Carbon Steel |
0.210 |
0.450 |
0.600 |
0.025 |
0.045 |
0.022 |
98.00 |
|
Medium Carbon Steel |
0.400 |
1.00 |
0.60 |
0.06 |
0.025 |
0.025 |
96.30 |
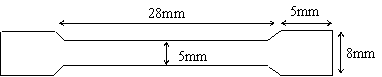
Figure 1. Tensile test specimen
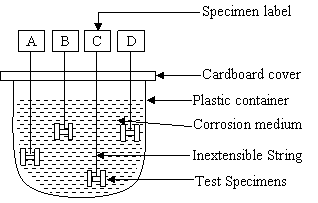
Figure 2. Experimental set-up showing immersion of steel samples in corrosion media
Results and Discussion
Corrosion behavior of low and medium carbon steels in cocoa and cassava extracts
Figure 3 shows the compares of the
weight loss of low and medium carbon steels in cocoa extract for sixteen days
of exposure.
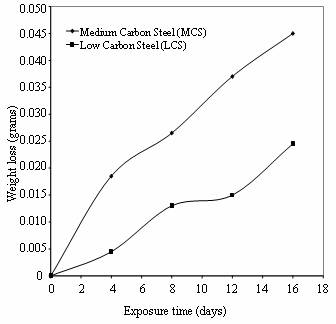
Figure 3. Weight loss Vs Exposure time of low and medium carbon steels in cocoa extract
For the first eight days of the exposure period, it was observed that the cocoa medium has turned milky, due to fermentation process and the same phenomenon was observed in cassava juice during the immersion period. It is evident from the plots that the corrosion rate of medium carbon steel is greater than that of low carbon steel throughout the exposure period. The same observation is noticed in cassava extract as shown in Figure 4.
This finding could be attributed to the higher carbon content in medium carbon steel as compared to low carbon steel (Robert, 2003). Figure 5 reveals the relative corrosion rates of the two steels in the cassava and cocoa media. It can be observed that low carbon steel in cassava show the highest resistance to corrosion at the same exposure period while high carbon steel in cocoa extract has the least corrosion resistance.
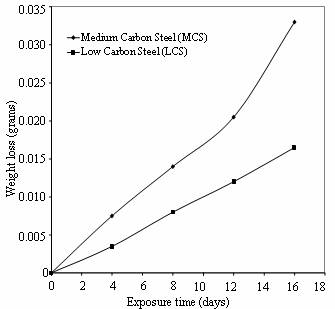
Figure 4. Weight loss Vs Exposure time of low and medium carbon steels in cassava extract
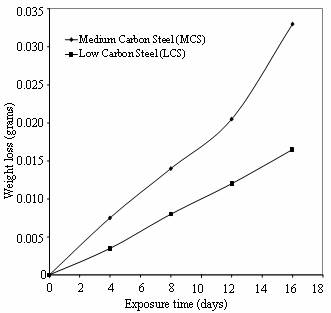
Figure 5. Corrosion behavior of low and medium carbon steels in cocoa and cassava extracts
The presence of carbonhydrates and fats in cassava extract (Akindahunsi, 1999 and Akindunmila and Glatz, 1998) are contributing factors for the corrosion of these metals. These constituents are sources of oxygen which may increase the oxidation process in the medium thus promoting corrosion of the materials. The presence of cyanogenic glucosides which could have been hydrolysed to hydrocyanic acid during fermentation is also a possible cause of corrosion of these materials in cassava extract (Oboh and Akindahunsi, 2003). Despite the reported anti-oxidation properties of cocoa products [Ziegleder and Sandmeir, (1982), Basaga et al, (1997), Azizah et al, (1999), Arts et al, (1999) and Osman et al, (2004)], it extract is observed to aid the corrosion of these steels. This effect is mainly due constituent of these steels as reflected in the higher corrosion rate in medium carbon steel.
SCC behavior of low and medium carbon steels in cocoa and cassava extracts
Figures 6 and 7show the SCC of low and medium carbon steel in cocoa and cassava extracts respectively expressed in terms of percentage elongation versus exposure period. The increase in length during extension is the difference in the gauge length of the tensile specimen at the start and completion of the constant extension rate test. This is represented as the percentage ratio of the elongation obtained and the initial gauge length of the metals. It was taken into cognizance that if the extension rate on the specimens is too high, the specimens would fail only under mechanical loading while the corrosion media under study would play little or no role in the cracking process. Also, too low strain rate may enable passive film to form over a long period of strain (Beaver and Koch, 1994 and SinghRaman and Muddle, 2004). Therefore, a typical NACE (McIntyre and Dillon, 1985) recommended strain rate of 3.0 X 10-7 s-1 was employed for all the constant extension rate tests.
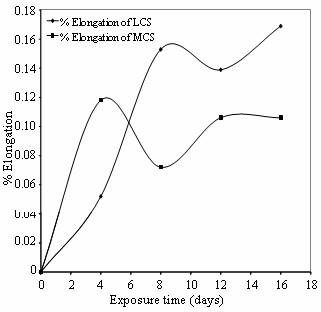
Figure 6. % Elongation Vs Exposure time of low and medium carbon steels in cocoa extract
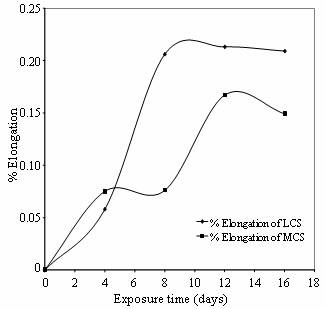
Figure 7. % Elongation Vs Exposure time of low and medium carbon steels in cassava extract
It is observed in these Figures that low carbon steel produced the higher extension than medium carbon steel throughout the exposure period studied. This behavior is partly due to the carbon contents of these steels and partly due to residual stresses in these metals brought about by the hardening effect by quenching process carried out on them. Quenching is a hardening process which is usually associated with brittleness in steels. The higher the carbon contents the more the brittleness in hardened steels. This further explains why medium carbon steel has lower extension than low carbon steel in the two media. Although the two materials seem to have a ductile mode of fracture, it is evident that low carbon steel produced more necking, which is the characteristic of ductile materials, than medium carbon steel throughout the exposure period. This is perhaps because low carbon steel also responds to tempering more quickly than medium carbon steel due to faster rate of atom (particularly carbon) re-organization during the process, hence equal tempering time of both steels would not produce equal ductility. Therefore the excess internal stresses that could not be relieved within these metals initiated cracks which are propagated in the presence of the agro-fluid media. The rate and extent of this propagation depend largely on the degree of corrosiveness of the constituents of these agro-effluents. The deduction infers that carbon as well as the agro-fluids media plays major role in the cracking propagation process of these steels.
This mechanism is further buttressed in
Figures 8 and 9 which express SCC in terms of percentage reduction in area.
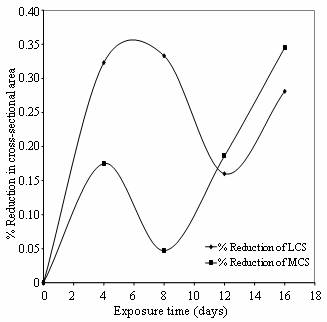
Figure 8. % Reduction in cross-sectional area Vs Exposure time of low and medium carbon steels in cocoa extract
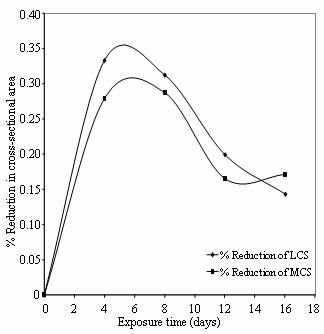
Figure 9. % Reduction in cross sectional area Vs Exposure time of low and medium carbon steel in cassava extract
The reduction in area is the difference in cross-sectional area of the gauge section of the test specimen at the start and completion of constant extension rate test. It is represented as the percentage ratio of normal projection area of the gauge section. The SCC susceptibility in these steels was enhanced by the presence of oxalic and hydrocyanic acids in cassava medium and citric acid in cocoa medium. The hydrogen ions of these acids caused an increase in amount of atomic hydrogen that can enter the lattice of the metals and embrittle the materials. This explains the role of hydrogen in cracking mechanism of low and medium carbon steels in these media.
Although fractographic analysis was not carried out on the failed
parts, it is suspected that intergranular cracking
propagation occurred over a considerable area of the fracture surface. In all
the constant extension rate tests carried out, the time to fracture was
observed to be faster in medium carbon than low carbon steel in the two media.
The tempered SCC susceptibility indexes which are percentage elongation and
percentage reduction in cross-sectional area are observed to decrease by
decreasing the constant extension. The above findings confirm the more common
hydrogen induced cracking mechanism during SCC.
Conclusions
The following conclusions could be drawn on the studies undertaken on the corrosion and SCC of low and medium carbon steels immersed in cocoa and cassava effluents.
1. Both low and medium carbon steels are susceptible to corrosion in both cassava and cocoa extracts. Low carbon steel is more resistance to corrosion than medium carbon steel in both cassava and cocoa extracts throughout the exposure period studied. The susceptibility of these steels to corrosion is attributed to the aggressive constituents present in these media.
2. Medium carbon steel is more susceptible to SCC than low carbon steel in both cassava and cocoa extracts; the susceptibility of these steels to SCC is mainly attributed to the presence of oxalic and hydrocyanic acids in cassava medium and citric acid in cocoa extract.
3. SCC is not only caused by hydrogen induced cracking but by carbon content in the steels which plays a major role in crack propagation process.
References
[1] Akindahunsi A. A., Oboh G., Oshodi A. A., Effect of Fermenting Cassava with Rhizopus Oryzae on the Chemical Composition of its Flour and Gari, Riv. Ital. Sostanze Grasse, 76, 1999, p. 437-440.
[2] Akindunmila F., Glatz B. A., Growth and Oil Production of Apiotrichum Curvatum in Tomato Juica, Journal of Food Protection, 6(11), 1998, p. 1515-1517.
[3] Ashassi-Sorkhabi H., Ghalebsaz-Jeddi N., Hashemzadeh F., Jahani H., Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Some Polyethylene Glycols, Journal of Electrochimica Acta 51, 2006, p. 3848-3854.
[4] Arts I. C. W., Hollman P. C. H., Kromhout D., Chocolate as a Source of tea Flavonoids, Lancet 354, 1999, p. 488-495.
[5] Azizah A. H., Nik Ruslawati M. N., Swee Tee T., Extraction
and Characterisation of Antioxidant from
[6] Basaga H., Tekkaya C., Acikel F., Antooxidation and Free Radical Scavenging Propertoes of Rosemary Extract, Lebensmittel-Wissenschaft und-Technologis 30, 1997, p. 105-108.
[7] Beavers J. A., Koch G. H., Limitations of the Slow Strain Rate Test for Stress Corrosion Cracking Testing, MTI Publication 39, 1994.
[8] Casales M., Salinas-Bravo V. M., Martinez-Villafane A., Gonzalex-Rodriguez, Effect of Heat Treatment on the Stress Corrosion Cracking of Alloy 690, Journal of Materials Science and Engineering A 332, 2002, p. 223-230.
[9] Chen X. H., Chen C. S., Xiao H. N., Cheng F. Q., Zhang G., Yi G. J., Corrosion Behaviour of Carbon Nanoyubes-Ni Composite Coating, Journal of Surface and Coating Technology 191, 2005, p. 351-356.
[10] Conn E. E., Cyanogenic Glucosides, Journal of Agricultural Food Chemistry 17, 1969, p. 519-526.
[11] Daret J., Paine J. P., Partridge M., Model Boiler testing to Evaluate Inhibitors for Caustic Induced Stress Corrosion Cracking of Alloy 600 Tubes, Proceedings of Seventh International Synposium on Environmental Degradation of Materials in Nuclear Power Systems - Water reactors, Breckenridge, Colorado, 1995.
[12] Dawson L. E., Gartner R., Lipid Oxidation in Mechanically Deboned Poultry, Journal of Food Technology 38, 1983, p. 112-116.
[13] Hahn H. P. S., Bummel M., Bowwy N. K., Becken K., Determination of Tannins and Their Correlation with Chemical and Protein Precipitation Method, Journal of Science Food Agriculture 61, 1993, p. 161-185.
[14] Jabeera B., Shibli S. M. A., Anirudhan T. S., Synergistic Inhibitive Effect of Tartarate and Tunstate in Preventing Steel Corrosion in Aqueous Media, Journal of Surface Science 252, 2006, p. 3520-3524.
[15] Le H. H., Ghali E., Stress Corrosion Cracking of Carbon Steel in Caustic Aluminate Solutions of the Bayer process, Journal of Corrosion Science 35, 1993, p. 435-442.
[16] Lehninger A. L., Bioenergetics and Metabolism, Principle of Biochemistry, 2nd Preprint, CBS, 1987.
[17] Lumsden J., Jeanjaguet S., Paine J. P. N., Mcilee A., Mechanism and Efectiveness of Inhibitors for SCC in a Caustic Environment, Proceedings of Seventh International Symposium on Environmental Degradation of Materials in Nuclear Power Systems- Water Reactors, Breckenridge, Colorado, 1995.
[18] McIntyre D. R., Dillon C. P., Guidelines for Prevention Stress Corrosion Cracking in the Chemical Process Industries, MTI Publication 15, 1985.
[19] Miglin M., Monter J., Wade C., Dombrowski-Psaila M., Mcilree A., SCC of Alloy 600 in Complex Caustic Environments, Proceedings of Seventh International Symposium on Environmental Degradation of Materials in Nuclear Power Systems- Water Reactors, Breckenridge, Colorado, p. 277, 1995.
[20] Moerck K. E., Ball H. R., Lipid Oxidation in Mechanically Deboned Chicken Meat, Journal of Food Science 39, 1974, p. 876.
[21] Osman H., Nazaruddin R., Lee S. L., Extract of Cocoa (Theobroma Cacao L) Leaves and their Anti-Oxidantion Potential, Journal of Food Chemistry 86, 2004, p. 41-46.
[22] Robert E. M., Effect on Marine Immersion Corrosion of Carbon Content of Low Alloy Steels, Journal of Corrosion Science 45, 2003, p. 2609-2625.
[23] Rondelli G., Vicentini B., Sivieri E., Stress Corrosion Cracking of Stainless Steels in High Temperature Caustic Solutions, Journal of Corrosion Science 39, 1997, p. 1037-1049.
[24] Rogante M., Battistella P., Cesari F., Hydrogen Interaction and Stress Corrosion Cracking in Hydrocarbon Storage Vessel and Pipeline Weldings, International Journal of Hydrogen Energy 31, 2006, p. 597-601.
[25] Saxena A., SinghRaman R. K., Muddle B. C., Slow Strain Rate for Monitoring Cracking of Mild Steels for Vessels and Pipes for Processing Using Caustic Solutions, International Journal of Pressure Vessels and Piping 83, 2006, 399-404.
[26] SinghRaman R. K., Muddle B. C., Stress Corrosion Cracking of Vessels and Pipes for Alumina Processing in Aggressive Caustic Soultions, International Journal of Pressure Vessels and Piping 81, 2004, p. 557-561.
[27] SinghRaman R. K., Louklan A., Simon G. P., ACA Corrosion and Prevention, Conference Proceedings, Perth Australia, 2004.
[28] Sriram R., Tromans D., Stress Corrosion Cracking of Carbon Steel in Caustic Aluminate Soultions-Slow Strain Rate Studies, Journal of Corrosion 41, 1985, p. 381-385.
[29] Ziegleder G., Sandmeir D., Antioxidation Action of Cocoa, Zucerund Suesswarenwirtschaft 35, 1982, p. 217-222.