Synthesis and Polymerization of Pyrole Characterization of Polypyrole
Moulay Abderrahim EL MHAMMEDI, Latifa KINANI and Abedelilah CHTAINI*
Molecular Electrochemistry and Inorganic Materials Team, University Cadi Ayyad, Faculty of Science and Technology, Beni-Mellal, Morocco
elmhammedi@yahoo.fr, latifa_kinani@yahoo.fr, chtainia@yahoo.fr (*corresponding author)
Abstract
The anodic polymerization of pyrole (P) onto Iron and Copper electrodes gives a PP/Metal composite. Some attractive properties de PP/Metal composites are employed by cyclic voltammetry, potentiometry, impedance measurements and scanning electron microscopy SEM.
Keywords
Polymerization; Polypyrole; Iron; Copper.
Introduction
The considerable current interest in conducting polymer has led to a number of important applications [1-3]. Special attention is currently being given to the preparation of new conducting electrode coatings based on composite polymers [4]. It is expected that this strategy will improve the behavior of conducting polymers and will result in polymers designed for specific application. Diaz and coworkers [5] described the preparation of poly(vinyl chloride) (PVC)/polypyrole (PP) composite membranes, by electropolymerizing PP inside a PVC film on the electrode surface.
Buttry and Hirais groups [6-7] prepared poly(aniline) (PA)/ Nafion composites by electropolymerization within precast Nafion layers, while Penner and Martin [8] illustrated the advantages of Nafion-impregnated Gorotex membranes.
Recent attention has focused on the preparation of charge balence composites, via the electropolymerization of cationic conducting polymers in the presence of anionic polymers [9-10]. Large polyelectrolyte anions, such as poly ( Styrensulfonate) and poly (vinylsulfate) have thus been employed in connection with PP.
In this report we describe a new iron/polypyrole and copper/polypyrole composites coating, prepared by the electropolymerization of PP in iron and copper alloys.
Experimental
Electrochemical experiments were performed with a potentiostat/galvanostat model voltalab 10 PGZ 100 assisted by master 4 electrochemecal software. All potentiels were measured vs the Ag/AgCl reference electrode.
The electrochemical measurements were carried out in a three-compartment cylindrial glass cell. Prior to its modification the simples (iron and copper) were polished with a 0.05µm alumina slurry for 2min, rinsed with doubly-distilled water and sonicated in a water bath for 5 min.
Results and Discussion
Electrochemical polymerization of the polymers can be carried out by either potential step or potential sweep methods, using a typical coating solution 0.1M monomers in methanol (MeOH). In the cyclic voltammetric curves for the polymerization (Figs. 1and 2) respectively, in iron and copper, the anodic current is even bigger during the early reverse scan that during the forward scan, leading to a cross-over. These features indicate [11-12] that deposition of the polymer proceeds through a nucleation and growth mechanism as reported for other conducting polymers [12].
Typical cyclic Volta metric curves of polypyrole film in pure background electrolyte in iron and copper ample are shown in Figs. 1 and 2 respectively. After the initial oxidation of the polymer at 0.5 V a minor pre-peak can be observed between 0.8 and 1.2 in iron, and between -0.4 and -0.3 in copper.
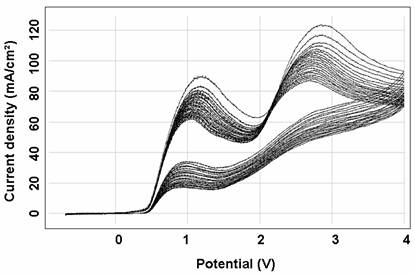
Figure 1. Cyclic voltammogram for electrochemical polymerization of 0.1M pyrole in 0.1M NaOH, at iron electrode, at 100 mv/s, 20 cycles.
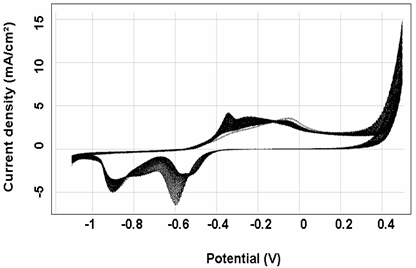
Figure 2. Cyclic voltammogram for electrochemical polymerization of 0.1M pyrole in 0.1M NaOH, at copper electrode, at 100 mV/s, 30 cycles.
The main peaks between 2 and 3 V for iron and between -0.2 and 0 in copper are followed by a big background current. The film is relatively stable in the bath samples (iron and copper) with repeated potential cycling. Also, the results of successively cyclic voltametry scan on the iron and copper electrodes indicated that the current peaks P1, P2 (Fig. 1) and P1, P2, P4, P5 (Fig. 2) of iron and copper respectively increased successively while P2 and P3 of iron and copper respectively decreased, and finally, all of them were unaltered. The decrease of P2 (Fig. 1) and P3 may attribute to the reductive product which concentrated on the surface of electrode. And the increase of current peak P1, P3 (Fig. 1) and P1, P2, P4, P5) may be ascribed to the electroactive species that concentrated on the electrode surface and it could be easily oxidized and reduced.
An impedance spectroscopy study was performed in order to confirm the results obtained by the cyclic voltammetric tests. Figs. 3 and 4. shown the impedance diagrams recorded respectively, for iron and copper with and without polymers. In the both samples (Iron and Copper), the impedance curves are in the form of half circle which can be attributed to the electron transfer step. The diameter of the circles increased considerably in iron/polymer and Copper/polymer electrodes, probably because the polymer over oxidized at such positive potentials it becomes electrochemically inactive and lead the increase of the electron transfer resistance.
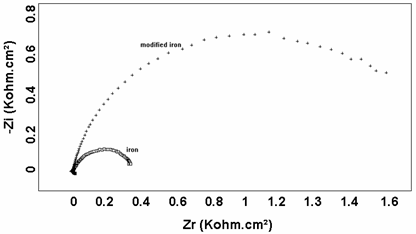
Figure. 3. Electrochemical Impedance Spectroscopy for iron (a) and iron/polypyrole (b) electrodes in 0.9% NaCl
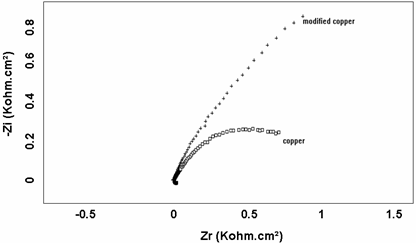
Figure 4. Electrochemical Impedance Spectroscopy for copper(a) and copper/polypyrole
(b) electrodes in 0.9% NaCl
Scanning electron microscopy of copper/polymer (Fig. 5) and iron/polymer (Fig. 6) showed the regular polypyrole films. In the cross-sectional view the periodicity of the multilayer can be observed.
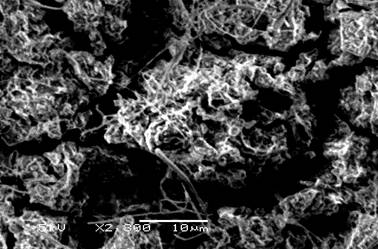
Figure 5. Scanning electron micrograph of Polypyrole/Iron.

Figure 6. Scanning electron micrograph of Polypyrole/Copper
Conclusion
The results presented in this article clearly demonstrate how the polymerization conditions and the type of electrode support influence the propreties of electrochemically prepared polymer modified electrode.
The experiments described above indicate that significant advantages can be achieved by anodic polymerization of pyrrole on iron and copper in an aqueous solution containing MeOH. The resulting composite electrode coating exhibits interesting proprieties in iron and copper.
References
1. Stotheim T. A., (Ed.), Handbook of conducting Polymers, Marcel Dekker, New York, 1986.
2. Dang S., Wang Y., Electroanalysis, 1989, 1, p. 99.
3. Seymour R. (Ed.), Conducting Polymers, Polenum Pron, New York, 1981.
4. Diaz F., Lacrois J. C., Synthesis of electroactive/conductive polymer films: electrooxidation of heteroaromatic compounds, New J. Chem., 1988, 12, p. 171-180.
5. Depaoli M. A., Waltman R. J., Diaz A. F., Bargon J., Molecular polymerpolymer compositions. Synthetic aspects, J. Chem. Soc. Chem. Commun., 1984, p. 1016-1020.
6. Ozata M., Butty O. A., Effect of gonatodropin and testotsterone treatments on prostate volume, J. Electroanal. Chem., 1988, 257, p. 71-79.
7. Hirai T., Kuwabata S., Yonegama H., Electrochemical behaviors of polypyrrole, poly-3-methylthiophene, and polyaniline deposited on nafion-coated electrodes, J. Electrochem. Soc., 1988, 135, p. 1132-1137.
8. Penner R. M., Martin C. R., Controlling the morphology of electronically conductive polymers, J. Electrochem. Soc., 1986, 133, p. 310-313.
9. Zhou O. X., Miller L. L., Valentine J. R., Manipulating and Monitoring Biomolecular Interactions with Conducting Electroactive Polymers, J. Electroanal. Chem., 1990, 261, p. 147-150.
10. Wang J., Golden T., Polishable and robust modified graphite epoxy electrodes, Anal. Chem., 1989, 61, p. 1337-1341.
11. Gunawardena G., Hille G., Montenegro I., Scharifker B., Nucleation and growth of Cu onto polycrystalline Pt electrode, J. Electroanal. Chem., 1982, 138, p. 225-228.
12. Asavapiriyanont S., Chandler G. K., Gunawardena G. A., Pletcher D., The electrodeposition of poly-N-methylpyrrole films from aqueous solutions, J. Electroanal.chem., 1984, 177, p. 245-251.