Electrochemical Studies of Paraquat Adsorbed onto Crystalline Apatite
Moulay Abderrahim EL MHAMMEDI and Abdelilah CHTAINI*
Molecular Electrochemistry and Inorganic Materials Team, University Cadi Ayyad, Faculty of Science and Technology, Beni-Mellal, Morocco.
elmhammedi@yahoo.fr, chtainia@yahoo.fr (* corresponding author)
Abstract
The carbon paste electrode (CPE) has been used to analyze the electrochemical behavior of paraquat (PQ) adsorbed onto synthesized hydroxyapatite phosphocalcique (HAP) in K2SO4 (0.1M). The cyclic voltammetry results obtained corrobate with square wave voltammetry. The influence of variables such as the concentration of paraquat adsorbed onto apatite (PQ/HAP), and the potential scan rate was tested.
X-ray diffraction analysis (XRD), Fourier transformed infrared spectroscopy (FTIR) analysis and inductively coupled plasma-atomic emission spectrometry (ICP, AES) were used for characterization of the apatite.
Keywords
Praquat (PQ); Hydroxyapatite (HAP); Carbone Paste Electrode (CPE); Adsorption.
Introduction
A wide number of analytical methods, based on the most commonly employed physico-chemical techniques for identification of organic compounds (UV, IR, HPLC, GC, MS) [1]-[5]. At present pollution of environment by pesticides is one of the main health risk factors [6],[7]. An effective control of maximum admissible concentrations of pesticides in water, soil, air, materials, and food is essential for improving the quality of life. Investigations aimed at the development of modern analytical techniques for pollutant detection are directed to wards increasing their sensitivity, specificity, simplicity and rapidity [8],[9]; the electrodes have demonstrated to be increasingly suitable for diverse analytical application [10]-[13]. On of their most claimed advantages is the possibility of performing measurements in highly resistive media.
The aim of the present paper is studies the electrochemical behavior of paraquat adsorbed onto hydroxyapatite phosphocalcique (HAP), at carbon paste electrode (CPE) in K2SO4 (0.1 M). Studies of cyclic voltammetry and amperometry were performed first to define the optimal experimental conditions of the electrochemical measurements.
In this paper, we describe the electrochemical analysis of paraquat on a carbon paste electrode. Paraquat adsorbs on the surface of the apatite and provides a facile voltammetric quantitative method for some electroactive paraquat. The electrochemical characterization of adsorbed electroactive paraquat compounds were evaluated using cyclic voltammetric and square-wave voltammetric (SWV) analysis.
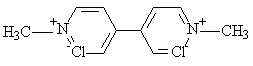
Paraquat molecular formulae.
Experimental
Preparation of apatite
The apatite was synthesized by doubly decomposition [14]. A calcium nitrate Ca (NO3)2.4H2O solution was added by slow rate (10 ml/min) into a boiling diammonium phosphate solution containing NH4OH. The pH of the mixture was about 10, and was maintained at this level throughout the reaction with a pH stat by addition of 3 vol % ammonia solution. After total addition of reactant the suspension was filtered in a large Buchner. The precipitate obtained was rapidly washed with deionized water and calcined at 900°C about 3h.
The powder prepared was characterized by X-ray diffraction analysis (XRD: Cu Kα radiation, XPERT) to evaluate the purity of the synthesized hydroxyapatite powder. The IR spectra of the speciments have been obtained on a PERKIN-ELMER FTIR) spectrophotometer from KBr pellets in order to determine hydroxyapatite stoechiometry deviations; in particular PO43- and/or OH- groups. In order to evaluate Ca/P ratio of powder, inductively coupled plasma-atomic emission spectrometry (ICP-AES) analysis was performed.
Adsorption measurements
The solution of paraquat 10-3 M was prepared by dissolving the appropriate amount of paraquat in 20 ml of ethanol, in glass bottles, at room temperature. The apatite (100 mg) was contacted for 5, 10, 15, 30, 50, 80, 120, and 1200 minutes with the paraquat. The suspension was centrifuged at 6000 rpm during 20 min, and the supernatant was analyzed to determine the concentration of paraquat by measuring the absorbance value of paraquat at 300 nm using Schimaddzu UV-161 UV-vis spectrophotometer.
Electrochemical measurements
The precipitate apatite contacted with paraquat for 1200 minutes, was added to the graphite powder to give a mixture that was (50%, 75%, 96% and 99%) apatite by weigh. The K2SO4 (1ml, 0.1 M) was then added and ground with the mixture (HAP, CPE); the obtained paste was packed into a home built electrode assembly
consisting of two concentric lengths of tubing in a pistonlike configuration. The geometric surface area of electrode was (0.1256 cm²). A bar of carbon inserted into carbon paste provided the electrical contact.
Cyclic voltammetric and SWV measurements were performed using an electrochemical analyzer voltalab Master 4 connected to a personal computer. The electrochemical measurements were performed using a three electrode system, including a mixture (HAP, CP) as the working electrode, platinum as a counter electrode and a saturated calomel reference electrode (SCE). The electrolytic solution (K2SO4, 0.1 M) was deoxygenated out at ambient temperature of the laboratory.
Results
Characterization of apatite
The XRD patterns of the prepared powders shown in Figure 1 reveal a crystalline apatite. No other phase has been detected.
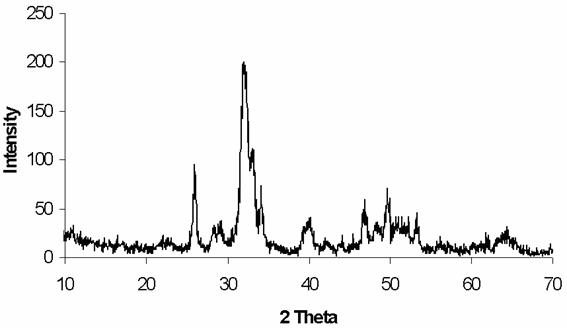
Figure 1. XRD pattern of the synthetic apatite
The IR spectra (Figure 2) showed bands characteristic of phosphocalcique. No spectral bands corresponding to carbonate ion were detected. All spectra show bands that can be assigned to phosphate groups, and hydroxyl in apatite environment [15]. The ICP analysis of synthetic apatite gives a Ca/P molar ratio of 1.667.
Adsorption onto apatite
The amount of paraquat adsorbed was obtained by subtracting the equilibrium concentration of paraquat from the initial concentration before the adsorption process.
The adsorption isotherms obtained for the paraquat are plotted in Fig. 3 as may be seen, that sharply rising adsorption virus time is indicate of high affinity between paraquat and adsorbent surface in ethanol.
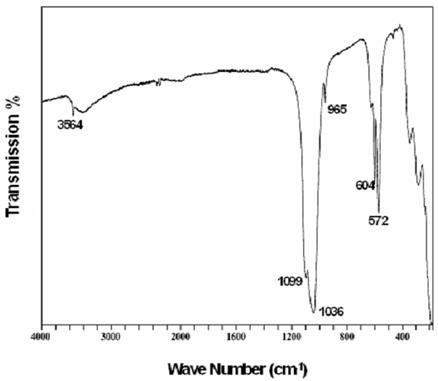
Figure 2. IR spectra of the synthetic apatite
Behavior of cyclic voltammetry
As shown in Fig. 4 on the carbon paste electrode, the cyclic voltammograms of paraquat adsorbed onto apatite in K2SO4 (0.1 M), demonstrated an two reversible system (P1/P4), which occur at approximately -700 mV and (P2/P3) observed at -100 mV.
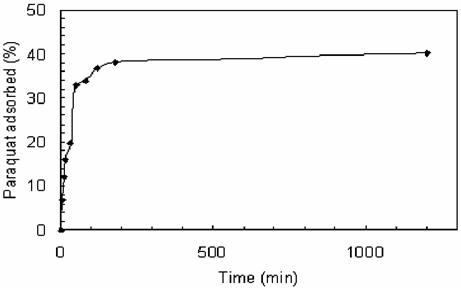
Figure 3. Percentage of adsorbed parquat onto apatite
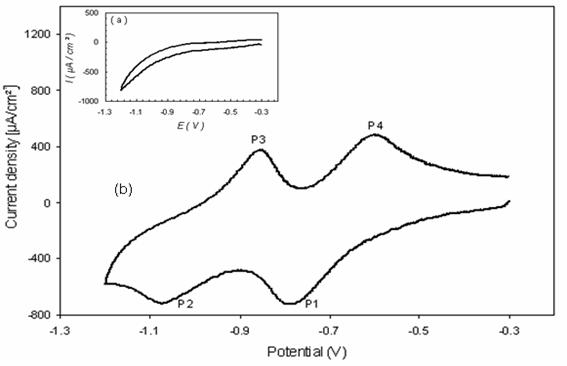
Figure 4. Cyclic voltammograms of (a) HAP, (b) paraquat adsorbed onto HAP,
at CPE in K2SO4 0.1M, PQ (HAP)/CP=0.5 at v=50 mV/s
The system (P1/P4) can be attributed to the redox couple [16]:
M2+ + 1 e- → M+
The second system represented by (P2/P3) is associated to couple [16]:
M+ + 1e- → M
Figure 5 shows, with increase of the scan number, the currents for both anodic and cathodic peaks decrease steadily, which indicates that the paraquat are eliminated continuously on the apatite surface.
The cyclic voltammograms corresponding to the response of paraquat adsorbed onto apatite at carbon paste electrode in 0.1 M K2SO4 solution at pH = 5.6 at different scan rate represented in Figure 6. As this figure, shows the anodic and cathodic peaks currents are linearly proportional to the scan rate. On the other hand, figure 7 shows that the currents for peaks increase continuously with increasing of an amount of PQ/HAP included with carbon paste (CP) into working electrode; to obtain a maximum at PQ (HAP)/CP about 96%.
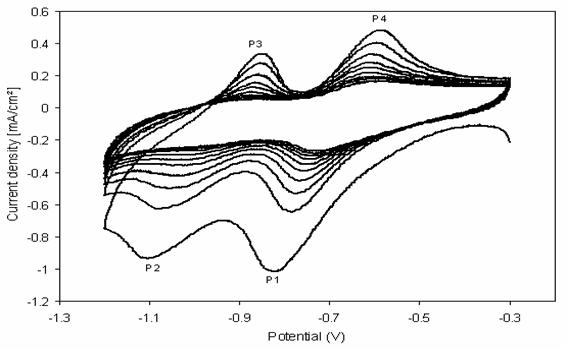
Figure 5. The cyclic voltammogram at CPE of paraquat adsorbed onto HAP, PQ (HAP)/CP=0.5 in K2SO4 0.1 M; at v=50 mV/s, 10 cycles
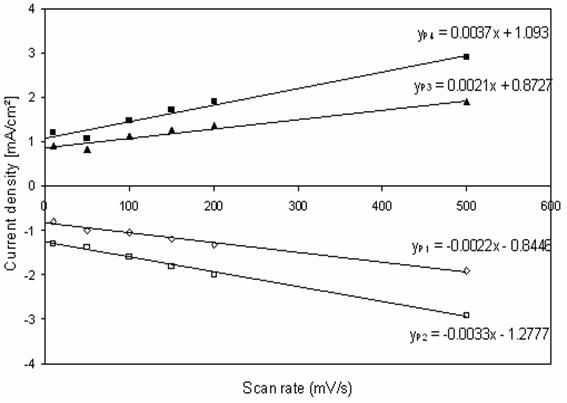
Figure 6. Dependence of the peak currents of paraquat adsorbed onto HAP
on the scan rate, at CPE in K2SO4. 0.1 M, PQ (HAP)/CP=0.9
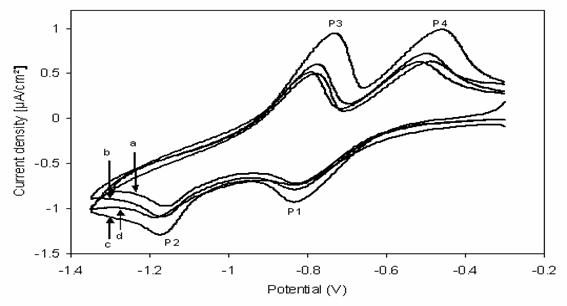
Figure 7. cyclic voltammetric response of paraquat adsorbed onto HAP at different ratio [PQ (HAP)/CP] included into working electrode; (a) 0.50, (b) 0.75, (c) 0.96, (d) 0.99
Square wave voltammetry
Figuree 8 shows the square wave voltammetry (SWV) of paraquat adsorbed onto apatite (PQ/HAP) at carbon paste electrode (CPE). Here peak (P1) was observed at -700 mV and peak (P2) at -1000 mV virus SCE. Which is in chose agreement with values obtained in cyclic voltammetric. And we found that the peak potential on the carbon paste electrode almost not change with increase or decrease of PQ(HAP)/CPE ratio comparing with a potential obtained in cyclic voltammetric. The reductive peak increased along with the increase of the amount of PQ (HAP) inserted in the carbon paste; to gives a maximum at 96% of PQ (HAP) inserted.
The following calibration equation at – 700 mV, was obtained i (µA) = 47.166 + 1.436 PQ (HAP)/CP.
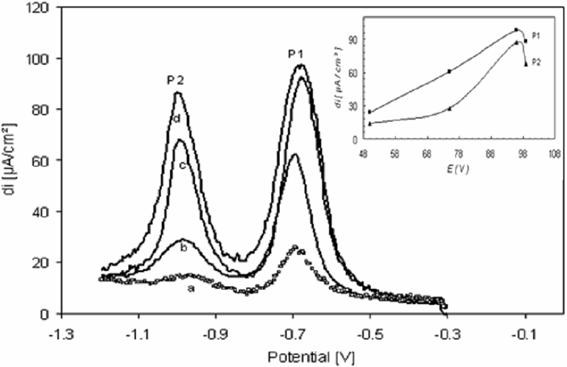
Figure 8. Square wave voltammograms at CPE in K2SO4 (0.1M), for paraquat adsorbed onto HAP at different ratio PQ (HAP)/CP; (a) 0.50, (b) 0.75, (c) 0.96, (d) 0.99
Chronoamperometric studies
The influence of accumulation time on the peak currents of paraquat adsorbed onto apatite was investigated. Figure 9 shows the chronoamperograms that were obtained for a series of PQ (HAP)/CP ratio. The results show that an increase of PQ (HAP)/CP was companied by an increase in cathodic current obtained for a potential step of -700 mV vs. SCE.
Calibration curves were recorded at 50 sec, in 0.1 M K2SO4 with a PQ (HAP)/CP ratio between 0.50 to 0.99 (fig. 9). It found that a maximum is obtained when PQ (HAP)/CP isabout 0.96. The following calibration equation was obtained i (mA/cm²) = 0.1241 - 0.0026 PQ (HAP)/CP.
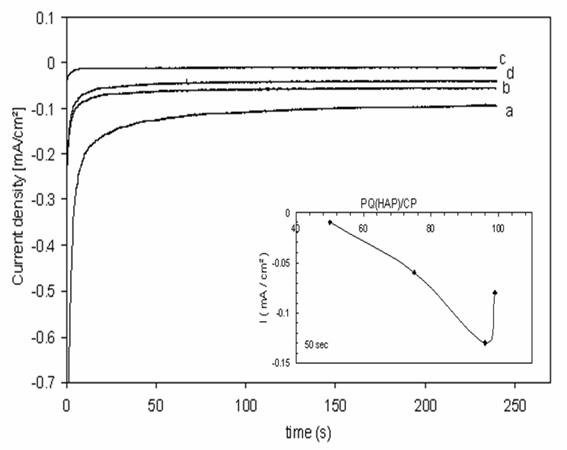
Figure 9. chronoamperometric response of paraquat adsorbed onto HAP,
at different ratio PQ (HAP) /CP, at CPE in K2SO4 0.1 M
Conclusion
In conclusion, the hydroxyapatite phosphocalcique used in this study is a good support to determine the electrochemical behavior of the organic compounds (PQ) at carbon paste electrode.
A sensitive method for electrochemical analysis of paraquat has been demonstrated. This electrode in a square wave voltammetry and a voltammetric measurement was sufficiently sensitive to quantifying paraquat adsorbed onto apatite.
References