Effect of Rice Husk and Diatomite on the Insulating Properties of Kaolin - Clay Firebricks
Emmanuel Ogo ONCHE, Benjamin Iyenagbe UGHEOKE, Sunday Albert LAWAL and Utibe Martin DICKSON
Mechanical Engineering Department, Federal University of Technology, Yola, Nigeria
oncheogo@yahoo.com, ugheokeb@gmail.com, lawalbert2003@yahoo.com, princeutibe@yahoo.com
Abstract
This work was carried out to investigate the effect of rice husk and diatomite on the insulating properties of kaolin-clay firebrick. Five firebrick samples of different compositions were fired at 900°C, 1000°C, 1100°C, and 1200°C. Samples A-E are all insulating firebricks that can withstand temperatures ranging from 900°C to 1200°C since none of the samples crumbled during firing. The results showed that they all had good insulating characteristics with their highly porous structure making them suitable for backup insulation. Mixing ratios of 3:2:4:1 representing weight in grams of kaolin, plastic clay, rice husk and diatomite respectively for sample D gave the optimum performance values in terms of modulus of rupture, apparent porosity, apparent density, bulk density, and thermal conductivity at all temperatures. At 1200°C, the values are 22.57kgf/cm2 for modulus of rupture, 98.25% for apparent porosity, 2.38g/cm3 for apparent density, 1.11g/cm3 for bulk density, and 0.038w/mK for thermal conductivity.
Keywords
Refractories; Firebrick, Insulating; Conductivity.
Introduction
In an earlier paper [1], the properties of kaolin-rice husk insulating fire-bricks were identified. However, the use of rice husk to produce pores in insulating firebricks is not entirely sufficient, since on firing, it produces ash within the firebrick matrix. This ash actually decreases the efficiency of the firebrick.
As already reported [2], diatomite which is naturally highly porous and is found in large deposits in some States in Northern Nigeria, could be considered for use to improve the characteristics of kaolin clay firebricks but they had never practically carried out this work. This paper investigates the effect of varying rice husk and diatomite contents on the insulating properties of kaolin clay firebrick.
Materials, Equipment and Methods
The materials used in this work include Kaolin, plastic clay, rice-husk, diatomite and water.
The equipment employed include jaw crusher, pulverizer, electric furnace, kiln, sieve, container, measuring tape, vernier caliper, measuring cylinder, weight balance, electric oven, pyrometric cone, thermal conductivity apparatus.
The Production of Insulating Firebricks Test Specimens
Insulating firebrick consisting of kaolin, plastic clay, rice husk, and diatomite was produced using the following processes:
· Refined kaolin and plastic clay were crushed and ground separately, and stored in respective labeled dry containers. The rice husk was screened and examined to ensure that no rice grain existed in the mass. It was ground and carefully sieved using a mesh of size 30, and also stored in a labeled dry container. Diatomite was also crushed and ground properly, and stored away in a labeled dry container.
· 30% Kaolin, and 12% plastic clay were measured out and mixed with varying percentage proportions of rice husk and diatomite (Table 1).
Wooden moulds of internal dimensions (7.5 × 3.7 × 1.5) cm were used for hand - moulding of the bricks. Test specimens were formed according to the composition of the mixture. The moulded mixtures were left to dry in the atmosphere, and thereafter, they were dried in an oven at 110°C.
Table 1. Composition of Brick samples by weight (Total weight = 1500g)
|
Sample Code |
Kaolin |
Plastic clay |
Rice Husk |
Diatomite |
|
A |
450 |
180 |
450 |
420 |
|
B |
450 |
180 |
555 |
315 |
|
C |
450 |
180 |
645 |
225 |
|
D |
450 |
180 |
750 |
120 |
|
E |
450 |
180 |
435 |
435 |
The dried bricks were finally fired in a furnace at temperatures of 900°C, 1000°C, 1100°C, and 1200°C. This firing process caused the burning out of the rice husk in the finished bricks.
The initial, original, fired, dried, and wet lengths and dry weights were noted and recorded.
Testing methods of the Insulating Firebrick Specimens
Tests were carried out on the bricks samples to determine their shrinkage, modulus of rupture, apparent porosity, water absorption, apparent density and bulk density. These tests include:
· Shrinkage Test
The drying shrinkage, firing shrinkage and the total shrinkage were calculated for each test specimen using the following formulae stated in [3,4]:
|
|
(1) |
|
|
(2) |
|
|
(3) |
|
|
(4) |
· Water Absorption, Bulk Density, Apparent Density, and Apparent Porosity Tests
The test specimens were dried to ensure water loss, and later fired in the electric furnace. Their fired weights were measured and recorded. They were allowed to cool and then immersed in water contained in a beaker and their weights noted. After sometime, the specimens were removed after which, their soaked weights were measured and recorded. The respective bulk density, apparent density, apparent porosity and percentage water absorption were calculated using the formulae given in [3,5]:
|
|
(5) |
|
|
(6) |
|
|
(7) |
|
|
(8) |
where: D = Weight of fired specimen, S = Weight of fired specimen in water, W = Weight of soaked specimen suspended in air.
· Modulus of Rupture
Modulus of rupture tests were performed. Here, each test specimen was dried, fired and placed alongside one another on the bearing edges of the compression machine. The loads at which the specimens failed were calculated from the relation given by [5]:
|
|
(9) |
where: l - The distance between bearing edges in centimeters, b - Width of the specimen in centimeters, t - Thickness of the specimen, w - Load at which the specimen failed.
Results and Discussion
The results obtained for the shrinkage tests are presented in Tables 2 while the physical analysis carried out on the samples yielded the results summarized in Tables 3.
Table 2. Shrinkage values at various temperature regimes
|
Sample code |
Original length (cm) |
Dry length (cm) |
Fried length (cm) |
Dry shrinkage % |
Fired shrinkage % |
Total shrinkage, % |
Temperature (°C) |
|
A |
5 |
4.84 |
4.7470 |
3.2 |
1.921 |
5.06 |
900 |
|
B |
5 |
4.87 |
4.7110 |
2.6 |
3.264 |
5.78 |
900 |
|
C |
5 |
4.88 |
4.7315 |
2.4 |
3.043 |
5.37 |
900 |
|
D |
5 |
4.88 |
4.5345 |
2.4 |
7.079 |
9.079 |
900 |
|
E |
5 |
4.85 |
4.8035 |
2.8 |
1.162 |
1.162 |
900 |
|
A |
5 |
4.88 |
4.6905 |
2.4 |
3.883 |
6.19 |
1000 |
|
B |
5 |
4.85 |
4.6235 |
3.0 |
4.670 |
7.53 |
1000 |
|
C |
5 |
4.90 |
4.6595 |
2.0 |
4.908 |
6.81 |
1000 |
|
D |
5 |
4.88 |
4.6135 |
2.4 |
5.461 |
7.73 |
1000 |
|
E |
5 |
4.85 |
4.6960 |
3.0 |
3.175 |
6.08 |
1000 |
|
A |
5 |
4.87 |
4.4570 |
2.6 |
8.480 |
10.86 |
1100 |
|
B |
5 |
4.86 |
4.5160 |
2.8 |
7.078 |
9.68 |
1100 |
|
C |
5 |
4.88 |
4.3365 |
2.4 |
11.137 |
13.27 |
1100 |
|
D |
5 |
4.86 |
4.4735 |
2.6 |
7.952 |
10.53 |
1100 |
|
E |
5 |
4.89 |
4.5360 |
2.2 |
7.239 |
9.28 |
1100 |
|
A |
5 |
4.82 |
4.5450 |
3.6 |
5.705 |
9.10 |
1200 |
|
B |
5 |
4.81 |
4.5500 |
3.8 |
5.405 |
9.00 |
1200 |
|
C |
5 |
4.80 |
4.5395 |
4.0 |
5.427 |
9.21 |
1200 |
|
D |
5 |
4.84 |
4.4980 |
3.2 |
7.066 |
10.04 |
1200 |
|
E |
5 |
4.90 |
4.6445 |
2.0 |
5.214 |
7.11 |
1200 |
Table 3. Physical Analysis at various temperature regimes
|
Sample code |
Modulus of rupture (kgf/cm2) |
% Apparent porosity |
% Water absorption |
Apparent density (g/cm3) |
Bulk density (g/cm3) |
Thermal conductivity, K (W/mK) |
Temperature (°C) |
|
A |
1.60 |
100.97 |
102.17 |
2.49 |
0.99 |
0.012 |
900 |
|
B |
0.79 |
102.88 |
102.28 |
2.49 |
0.99 |
0.009 |
900 |
|
C |
2.22 |
105.17 |
110.90 |
2.42 |
0.95 |
0.007 |
900 |
|
D |
10.24 |
105.95 |
112.52 |
2.42 |
0.95 |
0.003 |
900 |
|
E |
2.27 |
102.11 |
104.89 |
2.53 |
0.98 |
0.011 |
900 |
|
A |
1.30 |
100.04 |
102.12 |
2.46 |
0.98 |
0.031 |
1000 |
|
B |
5.73 |
102.68 |
105.52 |
2.39 |
0.97 |
0.015 |
1000 |
|
C |
4.08 |
104.64 |
109.93 |
2.38 |
0.95 |
0.009 |
1000 |
|
D |
15.04 |
104.02 |
112.91 |
2.35 |
0.94 |
0.004 |
1000 |
|
E |
4.71 |
95.09 |
90.70 |
2.48 |
1.05 |
0.034 |
1000 |
|
A |
7.32 |
90.49 |
82.62 |
2.42 |
1.10 |
0.029 |
1100 |
|
B |
9.85 |
92.99 |
86.93 |
2.37 |
1.07 |
0.025 |
1100 |
|
C |
4.43 |
94.48 |
89.70 |
2.37 |
1.06 |
0.017 |
1100 |
|
D |
18.23 |
103.03 |
106.28 |
2.33 |
0.97 |
0.005 |
1100 |
|
E |
5.19 |
86.43 |
76.11 |
2.45 |
1.14 |
0.036 |
1100 |
|
A |
9.20 |
94.68 |
89.68 |
2.35 |
1.06 |
0.020 |
1200 |
|
B |
9.54 |
94.71 |
89.96 |
2.35 |
1.06 |
0.012 |
1200 |
|
C |
18.40 |
98.22 |
96.19 |
2.29 |
1.02 |
0.007 |
1200 |
|
D |
22.57 |
98.25 |
96.53 |
2.29 |
1.02 |
0.006 |
1200 |
|
E |
4.61 |
88.79 |
79.85 |
2.38 |
1.11 |
0.038 |
1200 |
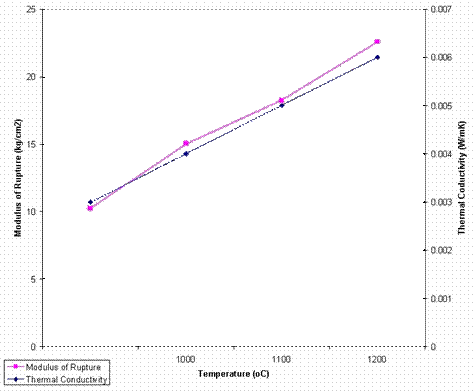
Figure 1. Variation of Modulus of rupture and Thermal conductivity with Temperature for optimum composition sample (Sample D)
Modulus of Rupture
At 900°C, sample B showed the lowest strength of 0.79 kgf/cm2 while sample D showed the highest strength of 10.24 kgf/cm2. At 1000°C, sample A, showed the lowest strength of 1.30 kgf/cm2, while D showed the highest strength of 15.04 kgf/cm2. At 1100°C, E showed the lowest strength of 5.19 kgf/cm2 while sample D showed the highest strength of 18.23 kgf/cm2, and at 1200°C, samples E and D showed the lowest strength of 4.61 kgf/cm2 and highest strength of 22.57 kgf/cm2 respectively.
It may be said that the lower the percentage of diatomite and the higher the percentage of rice husk in a sample, the higher its strength, and vice-versa. This is because diatomite creates empty pores in the firebrick matrix, while the rice husk leaves ash in the pores created. This left over ash is still capable of supporting and transferring load. The reduction in the number and size of pores of the brick samples due to increased rice husk percentage induce high transfer of load to the brick, thereby increasing their modulus of rupture.
The rising trend in the values of modulus of, rupture may also, not be unconnected with the formation of ceramic bonds which increases cold strength of a refractory. Modulus of rupture is observed to increase with increasing firing temperature. This, according to [6], is due to the replacing of the glassy matrix formed on cooling, with crystals that tend to interlock with each other giving rise to a strongly bonded mass, considerably increasing the refractoriness under load.
Apparent Porosity
At 900°C, sample A showed the lowest porosity of 100.97% while sample D showed the highest value of 105.95%. At 1000°C sample E showed the lowest porosity of 95.09% while sample C showed the highest porosity of 104.64%. At 1100°C, sample E shown the lowest porosity of 86.43% while D showed the highest porosity of 103.03%. Sample E showed the lowest porosity value of 88.79%, while sample B showed the highest porosity of 98.25%, at 1200°C.
Equal ratio of rice husk to diatomite showed least values of apparent porosity at all temperatures, but higher percentages of rice husk in the samples at all temperatures lead to higher porosity. According to [1], this is due to the burning out of rice husk during firing that leaves plenty of pores in a brick, thus making a brick porous, and a better heat insulator.
It is also noted that as temperature increases, the apparent porosity decreases. A possible explanation that could be given for this is that high temperatures tend to make the pores shrink.
Water Absorption
At 900°C, water absorption values of samples A and D were 102.17% and 112.52% respectively. At 1000°C, sample E showed the lowest value of 90.70% while sample D showed the highest value of 112.91%. At 1100°C, samples D and E showed the highest and lowest values of 106.28% and 76.11% respectively. Sample E showed the lowest value of 79.85% while sample D showed the highest value of 96.53%, at 1200°C.
It follows that as porosity increases, the rate of water absorption also increases.
As temperature increases, percentage water absorption rate decreases. This goes to buttress the argument that temperature increase leads to the shrinking of pores, which subsequently leads to the disappearing of the many pores the result of this, is that less water is absorbed by the moulds.
All compositions showed high porosity due to the presence of rice husk and diatomite resulting to high degree of water absorption.
Bulk Density
At 900°C, the bulk density of samples A and B showed the highest value of 0.99g/cm3 while samples D showed the lowest values of 0.95g/cm3. At 1000°C sample E showed the highest value of 1.05g/cm3 while D showed the lowest value of 0.94g/cm3. At 1100°C sample E showed the highest value of 1.14g/cm3 while D showed the lowest value of 0.97g/cm3. At 1200°C sample E showed the highest value of 1.11g/cm3 while C and D showed the lowest value of 1.02g/cm3.
The higher and lower percentages of rice husk and diatomite respectively in the samples led to lower values of bulk density. This is because rice husk burns out, allowing the volatile matter to escape, leading to weight drop and thus reducing its density.
Apparent Density
At 900°C, samples C and D showed the lowest values of apparent density of 2.42g/cm3 while sample B showed the highest value of 2.53g/cm3. At 1000°C, sample D showed the lowest value of 2.35g/cm3, while E showed the highest value of 2.48g/cm3. At 1000°C, samples D and E showed the lowest and highest values of 2.33g/cm3 and 2.45g/cm3 respectively. At 1200°C samples C and D showed the lowest values of 2.29g/cm3 each while sample E showed the highest value of 2.38g/cm3.
The obtained results prove that the higher the percentage of rice husk and the lower the percentage of diatomite in the brick samples, the lower their apparent density, in agreement with the conclusion given above.
Shrinkage
At 900°C, sample E showed the least shrinkage value of 3.93%, while sample D showed the highest value of 9.31%. At 1000°C, samples E showed the value of 6.08% while sample D showed 7.73%. At 1100°C sample E showed 9.2% while C showed the highest value of 13.27%. At 1200°C, sample E showed the least shrinkage of 7.11% while D showed the highest shrinkage age of 10.04%
It can be said that the higher the percentages of rice husk and the lower the percentages of diatomite, the higher the shrinkage.
Thermal Conductivity
Sample D had the lowest value of thermal conductivity of 0.003w/m.K, while sample A had the highest value of 0.012w/m.k at 900°C. At 1000°C, sample C and E had the lowest values of 0.004w/m.K and 0.034 w/m.K, respectively. Samples D showed the lowest value of 0.005w/m.K while E showed the highest value of 0.036w/m.K at 1100°C, at 1200°C, sample D showed the lowest value of 0.007w/m.K while E had the highest value of 0.038w/m.K
The results show that higher percentages of rice husk and lower percentages of diatomite induce low thermal conductivity to the samples. This is as a result of the increase of air volume obtained by the burning of the rice husk, a process which leads to pores forming within the samples to make them poor thermal conductors and hence, good backup insulators. From the results, it can be deduced that thermal conductivity decreases with decrease in density and increase in brick porosity.
Conclusions
Though the combination of rice husk and diatomite from the investigation carried out is suitable for use in the production of insulating firebricks, the quantity of diatomite must be kept as low as possible for optimum results. The relevance of diatomite in the composition is predicated on the fact that it improves the modulus of rupture of the formed brick, which is desirable in foundry applications.
Samples A - E are all insulating firebricks that can withstand temperatures ranging from 900°C to 1200°C and above. The highly porous structure of these bricks make them suitable for back up insulation since the air which fill the pores act as an insulator. The porosity of the samples in all compositions exceeded the standard 75% value for insulating firebricks as stated by [7].
Sample D gave the best results in terms of strength and thermal conductivity and its mixing ratio is therefore best suitable for use as a backup insulating firebrick.
Comparing the results of apparent porosity (88.79-98.25%), thermal conductivity (0.007-0.038w/mK) and modulus of rupture (4.61-22.57kgf/cm2) at 1200°C obtained from this work, with that of 61.83-95.93%, 0.005-0.134w/m K, and 4.26-19.10kgf/cm2) by [1], at the same temperature, it can be concluded that the combination of rice husk and diatomite improved the properties of the insulating firebricks.
Having observed from the results of this work that addition of diatomite improves modulus of rupture significantly in the insulating firebrick composition, and that this improvement trend increases with decreasing quantity of diatomite, it is imperative that the optimal value of diatomite in insulating firebricks composition be determined. Further work is therefore recommended in this regard.
References
1. Ugheoke B. I., Onche E. O., Namessan N. O., Asikpo G. A., Property Optimization of Kaolin - Rice Husk Insulating Fire - Bricks, LEJPT, 2006, 9, p. 167-178.
2. Adebayo A. A., Tukur L., Adamawa State in Map, Paraclete Publishers, Yola, p. 1-11, 1999.
3. Chesters J. H., Refractories: Production and properties, Iron and Steel Institute, London, p. 24-25, 1973.
4. Norsker H., The Self-reliant Potter: Refractories and Kilns, Friedr. Vieweg and Sohn Braunsweig /Weisbaden, 1987.
5. Chesti A. R., Refractories; production and properties, Iron and Steel Institute, London, p. 24-65, 1986.
6. Khanna O. P., Material Science and Metallurgy, Dhanpat Rai Publications, New Delhi, 2005.
7. Avallone E. A., Baumeister III T., Marks Standard Handbook for Mechanical Engineers, 10th ed., McGraw-Hill International Edition, New York, p. 6-153, 1996.