Synergistic Inhibition of Potassium Chromate and Sodium Nitrite on Mild Steel in Chloride and Sulphide Media
Ayo Samuel AFOLABI
School of Chemical and Metallurgical Engineering, University of the Witwatersrand, Johannesburg, South Africa & Metallurgical and Materials Engineering Department
Federal University of Technology, Akure, Nigeria
Abstract
The corrosion inhibition of mild steel in 3.5M sodium chloride and 0.3M sodium sulphide media using varied concentrations of potassium chromate and sodium nitrite was investigated in the study using the conventional weight loss measurement method. The results obtained show that potassium chromate produced a better inhibition performance than sodium nitrite in both media. This is attributed to cathodic and anodic inhibitive effects produced by the chromate ions as compared to only anodic inhibition in nitrite ions. Higher concentrations of these reagents also produced more inhibition in both environments under study. The combination of these inhibitors also produced an enhanced inhibition on the mild steel in the two media. This is attributed to synergistic action of these inhibitors which considerably reduced the cathodic and anodic activities thus promoting a continuous and protective film on the material within these media.
Keywords
Synergistic; Inhibition; Chloride and Sulphide Media
Introduction
Corrosion of metals is a major industrial problem that has attracted a lot of investigations in recent years [1 - 4]. Most metals are inherently unstable and have the natural tendency to react with their environments to obtain lower energy by forming a chemical compound in a more stable state. Steel materials which are very susceptible to attack in aggressive media are the commonly exposed metals in industrial environments [5]. Mild steel is particularly used in most structural shapes such as beams, plates, bars, and pipes used in seawater which contains chlorides and for conveying petroleum products that sometimes contain sulphides that are detrimental to the steel.
The use of inhibitors is one method of corrosion prevention among others such as cathodic protection [6, 7], anodic protection [8], and coating [9]. National Association of Corrosion Engineers (NACE) editor described corrosion inhibitors as substances that when added to an environment decrease or slow down the rate of attack on the metallic material [10]. They can be divided into three: anodic, cathodic and mixed, depending on whether they interfere with the corrosion reaction preferentially at the anodic or cathodic sites or both are involved [11]. Inhibitors are being extensively employed to minimize corrosion of many metallic structures in various media. Among common inhibitors are chromates, nitrates, benzoates, phosphates and borates [12, 13]. Nitrite is being used as inhibition admixtures in concrete reinforcement [14 - 16].
This work is set to study, compare the inhibition performance of potassium chromate and sodium nitrite and investigate the synergistic effect of these inhibitors on mild steel immersed in chloride and sulphide media. Synergistic effect of inhibitors is a combined action of inhibitors greater in total effect than the sum of the individual effects [17, 18]. According to Kalman et al, [19], cited by [18], synergism of corrosion inhibitors is either due to the interaction between components of inhibitor composition or due to interaction between the inhibitor and one of the ions present in the aqueous solution. Synergistic inhibition has been proved by several authors as an effective means to improve the inhibitive force of inhibitor, to decrease the amount of usage, and to diversify the application of inhibitor [20 - 23].
Material and Method
The chemical composition of the 15mm diameter mild steel used in this work is presented in Table 1. Fourteen corrosion test samples of the steel were prepared as shown in Figure 1. They were totally immersed (as shown in Figure 2) in various concentrations of sodium chloride and sodium sulphide containing different concentrations of potassium chromate and sodium nitrite inhibitors as shown in Table 2. Weight loss measurements were conducted for all the samples in the media using a sensitive electronic weighing machine for forty days following the procedures and precautions described by Bastidas et al, [24], Kolman et al, [25], and Ashassi-Sorkhabi et al, [26].
Table1: Chemical composition of the mild steel sample
|
Element |
C |
Si |
Mn |
P |
S |
Cu |
Fe |
|
% Composition |
0.140 |
0.220 |
0.500 |
0.040 |
0.040 |
0.045 |
balance |

Figure 1. Corrosion test sample
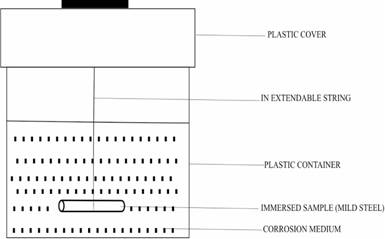
Figure 2. Experimental set-up showing immersion of the samples in corrosive media
Table 2. Concentrations of corrosion media and/or inhibitors
|
Symbol |
Concentration of Medium and/or Inhibitor |
|
A |
3.5M Sodium Chloride |
|
B |
0.3M Sodium Sulphide |
|
C |
3.5M Sodium Chloride and 0.5M Potassium Chromate. |
|
D |
3.5M Sodium Chloride and 1.0M Potassium Chromate |
|
E |
0.3M Sodium Sulphide and 0.5M Potassium Chromate |
|
F |
0.3M Sodium Sulphide and 1.0M Potassium Chromate |
|
G |
3.5M Sodium Chloride and 0.5M Sodium Nitrite |
|
H |
3.5M Sodium Chloride and 1.0M Sodium Nitrite |
|
I |
0.3M Sodium Sulphide and 0.5M Sodium Nitrite |
|
J |
0.3M Sodium Sulphide and 1.0M Sodium Nitrite |
|
K |
3.5M Sodium Chloride, 0.5M Potassium Chromate and 0.5M Sodium Nitrite. |
|
L |
3.5M Sodium Chloride, 1.0M Potassium Chromate and 1.0M Sodium Nitrite. |
|
M |
0.3M Sodium Sulphide, 0.5M Potassium Chromate and 0.5M Sodium Nitrite |
|
N |
0.3M Sodium Sulphide, 1.0M Potassium Chromate and 1.0M Sodium Nitrite |
Results and Discussion
Effect of NaNO2 and K2CrO4 inhibitors on corrosion
The effects of sodium nitrite and potassium chromate inhibitors on corrosion behaviour of mild steel in chloride and sulphide media are presented in Figures 3 and 4. It can be seen from the Figures that the weight losses of the samples decrease with the additions of sodium nitrite and potassium chromate inhibitors. Although corrosion occurred in all the samples, it was to a lower extent in the inhibited ones. This further confirms the findings of Fadayomi [27] that inhibitors do not totally stop corrosion but delays the onset of corrosion thereby decreases its rate in a corrosive environment. It is noticeable that better inhibition was obtained with potassium chromate than sodium nitrite inhibitor in the two media. It is also conspicuous that higher concentrations of the inhibitors produced a better inhibition effect on the steel in both media. This indicates that the higher the concentration of the inhibitor, the higher the inhibition activity, which correlates with the findings of Trethewey and Chamberlain [28] that at high concentration, anions may either become inhibitive or act in such a way as to plug any pores in a passive film.
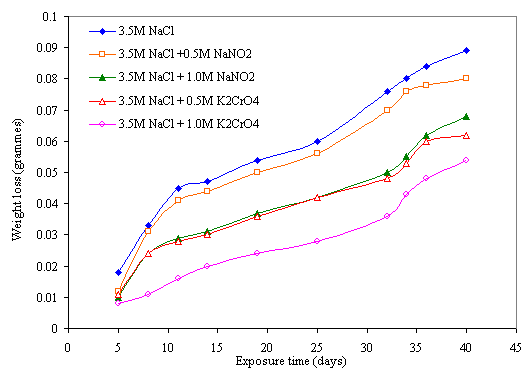
Figure 3. Effect of NaNO2 and K2CrO4 inhibitors on corrosion behavior of mild steel in 3.5M NaCl medium
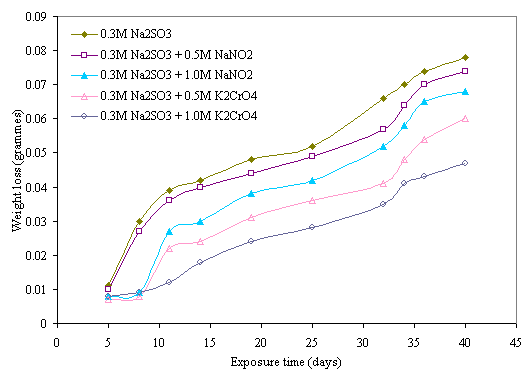
Figure 4. Effect of NaNO2 and K2CrO3 inhibitors on corrosion behavior of mild steel in 0.3M Na2SO3 medium
A good comparison of the two figures shows that better inhibition of the material occurred in sulphide medium than in chloride medium. This could be attributed to the aggressiveness of chloride ion which continuously breaks down the passivity produced by the inhibitors on the metal [28], as compared to the weak acidic nature of the sulphide medium.
The mild steel corrodes in air - saturated chloride and sulphide media as shown in Figures 3 and 4. Chromate is known to be an effective oxidizing anodic inhibitor which maintains the steel in the passive state thus preventing breakdown of the passive oxide which can lead to localized and/or uniform corrosion in chloride and sulphide media respectively. Chromate might have also acted as cathodic inhibitor which shows that acceleration of the cathodic reaction could be due to some catalytic action leading to the possible evolution of hydrogen, and not to the reduction of the chromate ion to the trivalent state CrO3-3. This catalytic effect on hydrogen evolution might be explained on the basis that chromate ion is absorbed on the surface of the steel and reduced to some intermediate valency e. g. tetravalent state, CrO4-4 which acts as mediator between the metal and water molecules as follows:
CrO2-4 + 2e = CrO4-4 (i)
CrO4-4 + 2H2O = CrO2-4 + H2 + 2OH- (ii)[29, 30]
This double inhibitive effect of chromate on the steel is clearly evident in higher inhibition obtained in chromate than nitrite inhibitor. This double protection action of chromate ensures the gradual cover of the surface of the metal with a protective film of chromium oxide. In the same vein, it can be observed that increase in concentration of chromate produces better inhibition. This could be attributed to the occurrence of the inhibition action at a certain concentration of chromate, and the attainment of a certain surface coverage of the steel with chromium oxide which increases with concentration of the inhibitor. It can be suggested that further reduction of the absorbed Cr4-4 to the trivalent state does not take place electrochemically, but proceeds through disproportionate of the tetravalent state. Also, at very low concentration the distances among the absorbed ions are too large to permit any chemical reaction resulting to surface passivation hence metal surface remain active.
Nitrite on the other hand, is an anodic inhibitor which functions at the anode and oxidizes ferrous ions to ferric ions thereby offers only single action inhibitive effect on the steel as reveals in the Figures [31]. The inhibitive effect also increases with increase in nitrite concentration which indicates that a higher concentration of this reagent is required to promote anodic passivity. This is in agreement with the findings of Rosenberg and Gaidis [32] in which the presence of hydroxide ion in the concrete created more passivating medium on the reinforced steel [16, 31].
Synergistic effect of sodium nitrite and potassium chromate
The effect of inhibitive action of sodium nitrite and potassium chromate mixture on corrosion of mild steel in chloride medium is shown in Figures 5 and 6. This indicates that better protection of the steel was obtained with the mixture of these reagents than with either of the inhibitors was used. Comparing the two figures reveals that more protection is also visible at higher concentration of the mixture of these inhibitors. The same features are conspicuous in Figures 7 and 8 in which the mixture of the two reagents gives a better inhibitive effect of mild steel in sulphide medium. However, better protection is evident in sulphide medium than chloride medium in all the samples either inhibited with single or mixture of the reagents.
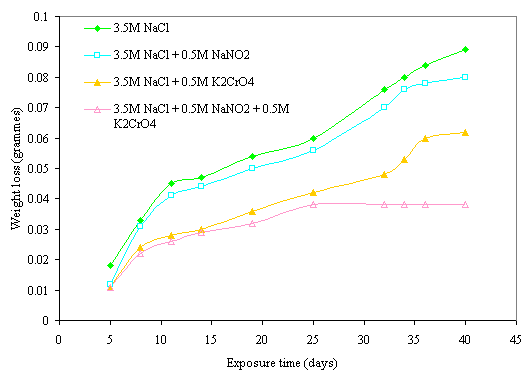
Figure 5. Effect of 0.5M NaNO2 and 0.5M K2CrO4 mixture on corrosion behavior of mild steel in 3.5M NaCl medium
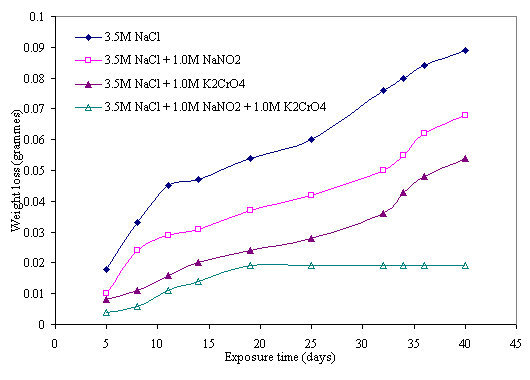
Figure 6. Effect of 1.0M NaNO2 and 1.0M K2CrO4 mixture on corrosion behavior of mild steel in 3.5M NaCl medium
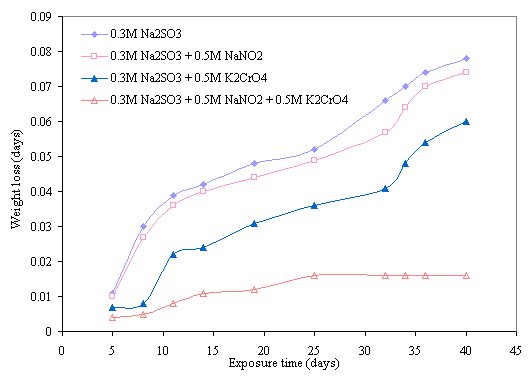
Figure 7. Effect of 0.5M NaNO2 and 0.5M K2CrO4 mixture on corrosion behavior of mild steel in 0.3M Na2SO3
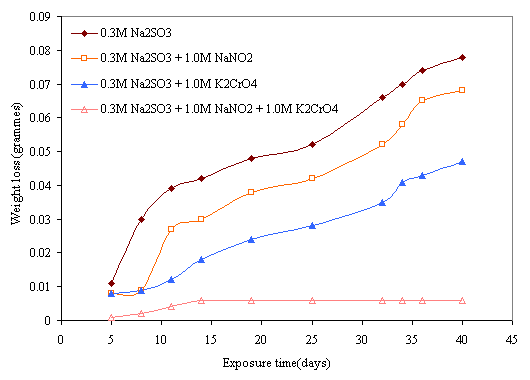
Figure 8. Effect of 1.0M NaNO2 and 1.0M K2CrO4 mixture on corrosion behavior of mild steel in 0.3M Na2SO3
The possible explanation for the better protection observed with mixture of the nitrite and chromate inhibitors can be attributed to synergistic action of these inhibitors on the steel. Synergistic effect is believed to have occurred as a result of cathodic inhibition provided by the chromate ion and anodic inhibition effected by the nitrite ion as previously discussed. The combined cathodic and anodic protection offered by these inhibitors provides a uniform protective film on the steel until there is film stability towards which is evident towards the latter days of exposure period. This film stability was obtained mainly due to synergism of the inhibitors as a result of continuous anodic and cathodic film repair effected by the inhibitive power of these inhibitors. This behaviors is similar to the findings of Oni [17] where a mixture of potassium chromate and sodium nitrate was used to obtain better inhibition of stress corrosion cracking (SCC) of low carbon steel in sulphuric acid. Similar results were also reported in recent research works of [20, 22, 23, 33, 34].
Conclusions
The following conclusions can be drawn from the investigation of the effect of inhibition performance of potassium chromate and sodium nitrite on mild steel in chloride and sulphide media.
1. Corrosion of mild steel in 3.5M sodium chloride is more than in 0.3M sodium sulphide. This is as a result of aggressive chloride ions in the former and weak acidic nature of the latter.
2. Potassium chromate produced a better inhibition performance of mild steel in 0.3M sodium sulphide medium than in 3.5M sodium chloride medium.
3. The higher the concentration of sodium nitrite and potassium chromate inhibitors, the more the inhibition performance on mild steel in the media studied.
4. The combination of sodium nitrite and potassium chromate inhibitors produced an enhanced inhibition performance on the mild steel in both 3.5M sodium chloride and 0.3M sodium sulphide media. This is due to the synergistic effect of the inhibitors on the steel within these media.
References
[1] Liu, G. Q, Zhu, Z.Y, Ke, W, Han, C.I. and Zeng, C. L., Corrosion. National Association of Chemical Engineers 57 (8), 2001, 730.
[2] Ilevbare, G.O. and Burstein, G.T., The Inhibition of Pitting Corrosion of Stainless Steels by Chromate and Molybdate Ions. Corrosion Science 45, 2003, 1545-1569.
[3] Munoz, A. I, Anton, J. G, Nuevalos, S. L, Guinon, J. L. and Herranz, V. P., Corrosion Studies of Austenitic and Duplex Stainless Steels in Aqueous Lithium Bromide Solution at Different Temperatures. Corrosion Science 46, 2004, 2955-2974.
[4] Cui, Z. D. Wu, S.L. Zhu, S.L. and Yang, X.J. Study on Corrosion Properties of Pipelines in Simulated Produced Water Saturated with Supercritical CO2. Applied Surface Science 252, 2006, 2368-2374.
[5] Craig S. Welding Technology Today Principle and Practices. Prentice - Hall, Inc. New Jersey. 1989, 301.
[6] Subasria,, R., Shinoharaa, T. and Morib, K. Modified TiO2 Coatings for Cathodic Protection Applications. Science and Technology of Advanced Materials 6, 2005, 501-507.
[7] Kim, D. K., Muralidharan, S., Ha, T. H., Bae, J. H., Ha, Y. C., Lee, H. G. and Scantlebury, J. D. Electrochemical Studies on the Alternating Current Corrosion of Mild Steel under Cathodic Protection Condition in Marine Environments. Electrochimica Acta 51, 2006, 5259-5267.
[8] Cecchetto, L., Delabouglise, D. and Petit, P., On the Mechanism of the Anodic Protection of Aluminium Alloy AA5182 by Emeraldine base Coatings Evidences of a Galvanic Coupling. Electrochimica Acta 52, 2007, 3485-3492
[9] Praveen, B. M., Venkatesha, T. V. Arthoba Naik, Y. and Prashantha, K. Corrosion Studies of Carbon Nanotubes–Zn Composite Coating. Surface & Coatings Technology 201, 2007, 5836-5842
[10] Nathan C. C., Corrosion inhibitors. National Association of Corrosion Engineers. (NACE) 1973, 279.
[11] Ramachandran, V.S., In: Concrete Admixtures Handbook: Properties, Science, and Technology. Park Ridge, NJ, USA: Noyes Publications, 1984, 540–545.
[12] Konno, H, Obayashi, S. K, Takahash,H and Nagayama, M., The Hydration of Barrier Oxide Films on Aluminium and its Inhibition by Chromate and Phosphate Ions. Corrosion Science 22, (10), 1982, 913-923.
[13] Isaacs, H.S, Virtanen, S. Ryan, M. P. Schmuki, P. and Oblonsky, L.J., Incorporation of Cr in the Passive Film on Fe from Chromate Solutions. Electrochimica Acta 47, 2002, 3127-3130.
[14] Montes, P. Bremner, T. W. and Lister, D. H., Influence of Calcium Nitrite Inhibitor and Crack Width on Corrosion of Steel in High Performance Concrete Subjected to a Simulated Marine Environment. Cement & Concrete Composites 26, 2004, 243-253.
[15] Sideris, K.K. and Savva, A.E., Durability of Mixtures Containing Calcium Nitrite Based Corrosion Inhibitor. Cement & Concrete Composites 27, 2005, 277-287.
[16] Ann a, K.Y. Jung, H.S. Kim, H.S. Kim, S.S. and Moon, H.Y., Effect of Calcium Nitrite-based Corrosion Inhibitor in Preventing Corrosion of Embedded Steel in Concrete. Cement and Concrete Research 36, 2006, 530-535.
[17] Oni, A., Inhibition of Stress Corrosion Cracking of a Low Carbon Steel in Sulphuric Acid by Potassium Chromate – Sodium Nitrate due to Synergism. Nigerian Journal of technical Education 14 (1), 1997, 93-100.
[18] Li, X. Deng, S. Mu, G. and Qu, Q., The Synergistic Inhibition Effect of Rare Earth Cerium (IV) Ion and Iso–Vanillin on the Corrosion of Cold Rolled Steel in 1.0 M H2SO4 Solution. Materials Letters, 2006a, Article in press.
[19] Kalman, E. Lukovits, I. and Palinkas, G., A Simple Model for Synergism of Corrosion Inhibitors. ACH – Models Chemistry 132 (40), 1995, 527-537.
[20] Ai, J. Z. Guo, X.P. Qu, J.E. Chen, Z.Y. and Zheng, J.S., Adsorption Behaviour and Synergistic Mmechanism of a Cationic Inhibitor and KI on the Galvanic Electrode. Colloids & Surfaces A: Physicochemical Engineering Aspects 281, 2006, 147-155.
[21] Bouklah, M, Hammouti, B, Aouniti, A. Benkaddour, M. and Bouyanzer, A., Synergistic Effect of Iodide Ions on the Corrosion Inhibition of Steel in 0.5 M H2SO4 by New Chalcone Derivatives. Applied Surface Science 252, 2006, 6236-6242.
[22] Li, X, Tang, L, Mu, G, Li, L. and Liu, G., The Synergistic Inhibition of the Cold Rolled Steel Corrosion in 0.5 M Sulfuric Acid by the Mixture of OP and Bromide ion. Materials Letters, 2006b, Article in press.
[23] Tang, L. Li, X. Mua, G. Li, L. and Liu, G., Synergistic Effect Between 4-(2 pyridylazo) Resorcin and Chloride Ion on the Corrosion of Cold Rolled Steel in 0.5M Sulfuric Acid. Applied Surface Science 252, 2006, 6394-6401.
[24] Bastidas, J. M. Fosca, C. Chico, B. and Otero, E., Weight Loss and Electrochemical Results for Two Super–Austenitic Stainless Steels in Chloride–Fluoride Mixtures. Corrosion Science 38 (3), 1996, 559-563.
[25] Kolman, D. G. Ford, D. K. Butt, D. P. and Nelson, T. O. Corrosion of 304 Stainless Steel Exposed to Nitric Acid-Chloride Environments. Corrosion Science 39, (12) 1997, 2067-2093.
[26] Ashassi-Sorkhabi, H. Ghalebsaz-Jeddi, N., Hashemzadeh, F. and Jahani, H., Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Some Polyethylene Glycols. Journal of Electrochimica Acta 51, 2006, 3848–3854.
[27] Fadayomi, J. Corrosion Inhibitors. Concrete 31, (8), 1997, 21-22.
[28] Trethewey, K.R. and Chamberlain, J., Corrosion for Science and Engineering. 2nd Edition, 1995, Edinburgh Gate Harlow, Essex CM20 2JE, England.
[29] Berzins, T. and Delahay, P., Theory of Irreversible Polarographic Waves - Case of Two Consecutive Electrochemical Reactions. Journal of American Chemical Society 75, 1953, 5716-5720.
[30] Delahay, P. and Mattax, C. C., Theory of Electrolysis at Constant Current in Unstirred Solution. III. Experimental Study of Potential - Time Curves. Journal of American Chemical Society 76, 1954, 874 – 878.
[31] Gaidis, J. M., Chemistry of Corrosion Inhibitors. Cement and Composites 26, 2004, 181-189.
[32] Rosenberg, A. M. and Gaidis, J. M., The Mechanism of Nitrite Inhibition of Chloride Attack on Reinforcing Steel in Alkaline Aqueous Environments. Materials Performance 18 (11), 1979, 45-48.
[33] Jabeera, B, Shibli, S. M. A. And Anirudhan, T. S., Synergistic Inhibitive Effect of Tartarate and Tungstate in Preventing Steel Corrosion in Aqueous Media. Applied Surface Science 252, 2006, 3520-3524.
[34] Li, X, Tang, L, Li, L, Mu, G. and Liu, G., Synergistic Inhibition between o-Phenanthroline and Chloride Ion for Steel Corrosion in Sulphuric Acid. Corrosion Science 48, 2006, 308-321.