Investigation of the Inhibitive Effect of Pyrazolo [3, 4-b] Pyridine on Corrosion of Stainless Steel in 1 M HCl Solutions
Moulay Abderrahim EL MHAMMEDI and Abdelilah CHTAINI*
Molecular Electrochemistry and Inorganic Materials Team, University Cadi Ayyad, Faculty of Science and Technology, Beni-Mellal, Morocco
elmhammedi@yahoo.fr, chtainia@yahoo.fr (* corresponding author)
Abstract
The purpose of this study to investigate the effect of pyrazolo [3-4-b] pyridine on the corrosion inhibition of stainless steel in 1.0M hydrochloric acid (HCl) by using the following methods: the weight loss method, the potentiodynamic polarization methods, and the electrochemical impedance spectroscopy.
It was found that the adsorption of inhibitor could prevent steel from weight loss and the adsorption accorded with the Langmuir adsorption, the corrosion protection could be explained by the adsorption of inhibitor and formation of a protective layer attached to the metal surface.
Keywords
Pyrazolo [3-4-b] Pyridine; Corrosion Inhibition; Adsorption; Stainless Steel
Introduction
The use of inhibitors is one of the most practical methods for protection against corrosion, especially in acidic media [1]. Hydrochloric acid solutions are widely used for the pickling, cleaning, etching of stainless steel. The dissolution rate of the metal is quite high and the inhibition of these solutions with organic compounds, which may retard the dissolution rate, is favored. Different compounds have been reported to inhibit the corrosion of stainless steel. Most of the well known acid inhibitors are organic compounds containing N, O atoms [2]. The c and it’s allay by different inhibitors in acid medium has been studied by several authors [3-7]. It has been reported that the adsorption of nitrogenous compounds occurs with aromatic rings parallel to the metal surface [8].
The synthesis of new organic molecules offers various molecular structures containing several heteroatoms and substituents. Their adsorption is generally explained by the formation of an adsorptive film of a physical or chemical character on the metal surface. In continuation of our work on development of organic compounds as acid inhibitors, we have chosen an organic inhibitor namely pyrazolo [3-4-b] pyridine A (Figure 1) with a view to study it’s inhibiting properties on corrosion of steel in 1.0M HCl.
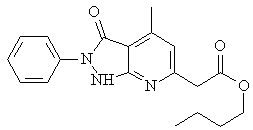
Figure 1. Molecular formulae of 2-(4-Methyl-3-oxo-2-phenyl-2, 3-dihydro-1H-pyrazolo
[3, 4-b] pyridin-4-yl) acetic acid butylester
In the present work, we investigate the corrosion of steel in 1.0 M HCl by inhibitor. Weight loss tests and electrochemical techniques (potentiodynamic and Rp polarization and impedance measurements) have been used to study the effect of addition of this compound on the corrosion of steel in hydrochloric acid solution.
Experimental
Inhibitor was synthesized by a previously described procedure [3]. The molecular structures are shown in Figure 1.
The aggressive solution (1 M HCl) was prepared by dilution of analytical grade 36% HCl with bidistilled water. The solutions were prepared by mixing HCl with the inhibitor. All tests have been performed in deaerated solutions and at room temperature (25°C). For electrochemical measurements, a conventional three-electrode glass cell with a platinum plat (1cm ´ 1cm) counter-electrode a saturated calomel electrode (SCE) as reference and Stainless steel as working electrode connected to a Voltalab 10 type computer controlled potentiostat. The working electrode surface (1cm²) mechanicals polished with emery paper, cleaned with distilled water and finally dried with filter paper. The electrochemical analysis involved, measuring of open circuit potential, gravimetric measurements, polarization studies and AC impedance in acidic media 1M (HCl).
Results
The open circuit potential of the stainless steel in 1M HCl with and without inhibitor solution is shown in Figure 2. With inhibitor the potential stabilized after 20 min at 0.5 V
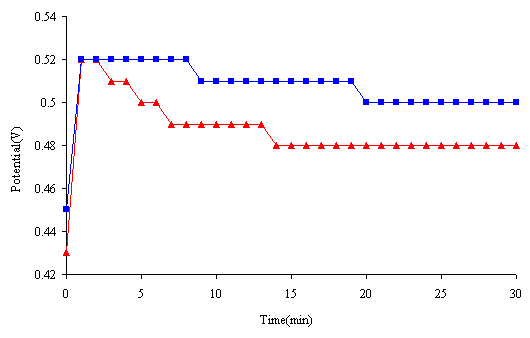
Figure 2. Open-circuit potential vs. time of the stainless steel in 1.0 M HCl solution. (▲) without inhibitor, (■) with 20mg/l of inhibitor
The polarization curves for steel in hydrochloric acid at various concentrations of inhibitor are shown in Figure 3.
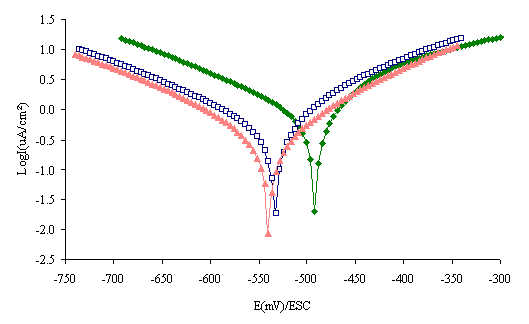
Figure 3. Polarization curves of steel in 1 M HCl with and without inhibitor at different concentrations. (♦) Without inhibitor, (□) 20mg/l, (▲) 60 mg/l
The electrochemical parameters determined from the polarization curves are given in Table1. The values of corrosion current density (Icorr) of stainless steel in the inhibited solutions were smaller than those for the inhibitor free solution.
Table 1. Electrochemical parameters from polarization measurements on stainless steel in 1MHCl without and with different inhibitor concentrations
|
Electrodes |
Blanc |
20 mg (inh) |
60mg (inh) |
|
E (i=0)/mV |
-492.1 |
-533.4 |
-539.3 |
|
Rp (W.cm2) |
28.36 |
47.75 |
72.47 |
|
Jcorr.(mA/cm2) |
0.728 |
0.464 |
0.416 |
|
Ba (mV/dec) |
104.5 |
107.7 |
131.1 |
|
Bc (mV/dec) |
-145.8 |
-145.9 |
-150.2 |
The corrosion behavior of stainless steel, in acidic media with and without inhibitor concentration was investigated by the ESC method at room temperature (Figure 4). The charge transfer resistance is calculated from the difference in impedance at lower and higher frequencies. The parameters derived from these investigations are given in Table 2. It found that, as the inhibitor concentration increases, the Rt increase, but the Cdl values tend to decrease, the decrease in the Cdl values is due to the adsorption of inhibitor on the metal surface.
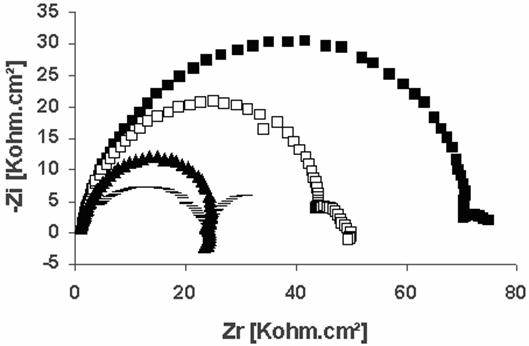
Figure 4. Electrochemical impedance spectra of steel in 1 M HCl with and without different inhibitor concentrations (-) Without inhibitor, (▲) 20mg/l, (□) 40 mg/l, (■) 80mg/l
Table 2. Nyquist diagrams for stainless steel in 1M HCL containing different concentrations of inhibitor.
|
Inhibitor |
Blanc |
20mg/l |
40mg/l |
80mg/l |
|
R1 (W. Cm2) |
0.833 |
1.226 |
1.859 |
1.411 |
|
R2 (W. Cm2) |
15.61 |
25.01 |
44.35 |
72.14 |
|
C (mF/cm2) |
57.8 |
402.1 |
452.1 |
348. |
The weight loss of steel in acidic media without and with inhibitor is determined after 30 h of immersion, the inhibition efficiency of inhibitor for the corrosion of stainless steel was calculated by using the following equation:
|
E %=( 1- Wcorr (inhb)/Wcorr) ×100 |
|
where Wcorr and Wcorr (inhb) are the weight loss for steel after immersion in acidic media without and in the presence of various concentration of inhibitor.
The parameters derived from this investigation are given in Table 3 and Figure 5.
Table 3. Inhibition efficiency obtained from the weight loss for different concentration of inhibitor in 1 M HCl.
|
Concentration of inhibitor (mg/l) |
Corrosion rate mg/cm2 h |
Inhibition efficiency E% |
|
0 |
2.9 |
- |
|
10 |
1 |
65.51 |
|
20 |
0.7 |
75.86 |
|
40 |
0.58 |
80.00 |
|
80 |
0.35 |
87.93 |
|
100 |
0.27 |
90.68 |
|
150 |
0.22 |
92.41 |
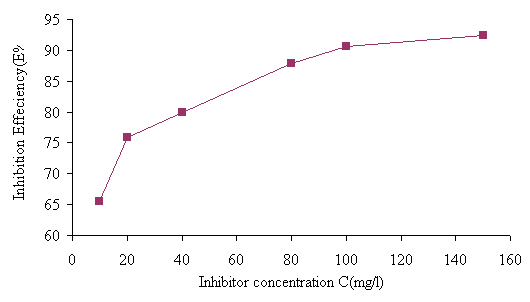
Figure 5. Inhibition efficiency for stainless steel in 1M HCl containing different inhibitor concentrations at room temperature
It is clear that inhibition efficiency increased with increasing inhibitor concentrations. The corrosion inhibition was caused by the adsorption of inhibitor on the metal surface in 1 M HCL; the degree of surface coverage (θ) for inhibitor in acidic media was evaluated from weight loss measurements using the relation [9].
|
θ = (Wo-W)/ (Wo-Wm) |
|
where Wm is the smallest weight loss.
Table 4 summarizes the values of θ of stainless steel in the presence of various concentrations of inhibitor at room temperature.
Table 4. The values of coverage obtained from the weight loss for different concentrations of inhibitor in 1 M HCl at room temperature
|
Inhibition Concentration (mg/l) |
0 |
10 |
20 |
40 |
80 |
100 |
150 |
|
Coverage (θ) |
0.00 |
0.70 |
0.82 |
0.86 |
0.95 |
0.98 |
1.00 |
|
C/θ |
- |
14.28 |
24.39 |
46.51 |
84.21 |
102 |
150 |
The values of θ increased with increasing inhibitor concentrations. The langmuir isotherm was applied to investigate the adsorption mechanism by the following expression:
|
θ = KC / (1+KC) |
|
one obtains
|
C/θ = 1/K + C (θ # 0) |
|
where C is the inhibitor concentration and K is defined as
|
K= (1/55.5) exp(-ΔGads/RT) |
|
C/θ virus inhibitor concentration at room temperature is shown in Fig. 6. The linear regression between C/θ and C was calculated, and K and ΔGads can be calculated according to the above equations. The result was shown in Table 5.
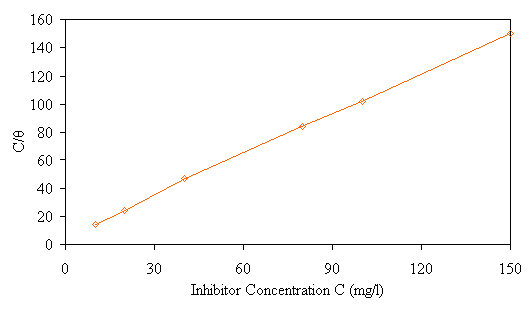
Figure 6. The relation between C/θ (mg/l) and C (mg/l) at room temperature
Table 5. Adsorption parameters of inhibitor on the steel surface at 25°C
|
Linear |
(correlation coefficient |
ΔGads (KJ/mol) |
|
0.969 |
4.5 |
13.678 |
It can be found that the correlation coefficients of straight line C/θ virus C of inhibitor approach 1 at room temperature. This illustrates that the aforementioned supposition is tenable on the whole, that is, the adsorption of inhibitor on steel surfaces conforms to Langmuirs adsorption isothermal equation.
The SEM images shown in Figure 7 provide an explanation for the phenomena. It is clear that the plate of stainless steel is uniformly covered with the protecting film. This observation clearly proved that the inhibition is due to the formation of an insoluble barrier film on the mild steel surface.
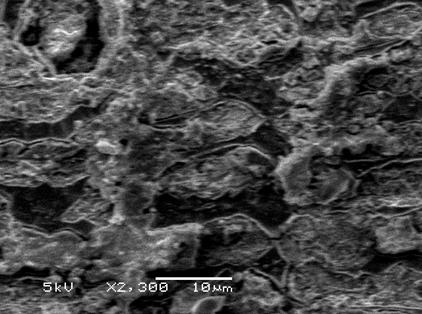
Figure 7. SEM photographs of the surface for stainless steel after immersion at 25°C
in 1 M HCl.
Conclusion
All investigated pyrazolo [3-4-b] pyridine A have shown good inhibiting properties for stainless steel corrosion in 1 M hydrochloric acid (HCl).
Adsorption mechanism of inhibitor on the stainless steel surface in 1.0M hydrochloric acid (HCl) was investigated. Assuming the decrease of the weight loss of stainless steel was caused by the adsorption of inhibitor on the steel surface and obeys the Langmuir equation.
References
1. Almaj M., Rawat J., Quraishi A., Infleunce of Polyamide Macrocyclic Compounds on the Inhibition of Corrosion of Mild Steel in Acid Solutions, Bulletin of electrochemistry, 1998, 14, p. 199-204.
2. Aouniti A., Hammouti B., Kertit S., Brighli M., The inhibition effect of some pyridines towards the corrosion of iron in hydrochloric acid solution, Bullet. of Electroch., 1998, 14, p. 193-199.
3. Gasparac R., Martin C., Investigations of the Mechanism of Corrosion Inhibition by Polyaniline. Polyaniline-Coated Stainless Steel in Sulfuric Acid Solution, J. Electrochem. Soc., 2001, 148, p. 4-11.
4. Agrawal A., Namboodhiri T., The inhibition of sulfuric acid corrosion of 410 stainless steel by thioureas, Corr. Science, 1990, 30, p. 37-44.
5. Ahmad N., MacDiarmid A. G., Inhibition of corrosion of stainless steels with the exploitation of conducting polymers, Synthetic Metals, 1996, 78, p. 2-13.
6. Luy C., Ives M. B., The improvement of the localized corrosion resistance of stainless steel by cerium, Corros. Sci., 1993, 34, p. 11-19.
7. Kilmartin P. A., Trier L., Corrosion inhibition of polyaniline and poly(o-methoxyaniline) on stainless steel, Synthetic Metals, 2002, 131, p. 1-7.
8. El-Rehim S., Refeay S., Taha F., Saleh M., Corrosion inhibition of mild steel in acidic medium using 2-amino thiophenole and 2- cyanomethyl benzoithiazole, J. of Applied Electrochemistry, 2001, 31, p. 4-8.
9. Rajappa S. K., Venkatesh T. V., Inhibition studies of a few organic compounds and their condensation products on the corrosion of zinc in hydrochloric acid medium, Turk. J. Chem., 2003, 27, p. 189-196.