The Inhibition Effect of Heterocyclic Compounds towards the Corrosion of Iron in Phosphoric Media
Khadija HNINI1, Salah FADEL2, Molay Abderrahim EL MHAMMEDI1, Abdelilah CHTAINI1, * and El Mostapha RAKIB2
1 Molecular Electrochemistry and Inorganic Materials Team, University Cadi Ayyad, Faculty of Sciences and Technology, BP: 523, Beni Mellal, Morocco
2 Analytical and Organic Chemistry Team, University Cadi Ayyad, Faculty of
Sciences and Technology, BP: 523, Beni-Mellal, Morocco
E-mails: chtainia@yahoo.fr; mhammedi@yahoo.fr;sfadel@yahoo.com; mrakib@yahoo.fr
* Corresponding author: Pr. A.Chtaini, Fax: 0011223485201, chtainia@yahoo.fr
Abstract
The behaviour of the 316 stainless steel in polluted phosphoric acid, has been studied in absence and presence of [(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-phenyl-methyl]-urea (HPU1) and [(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-(4-methoxy-phenyl)-methyl]-urea (HPU2) at different concentrations, Potentiodynamic and electrochemical impedance measurements showed that these compound act as cathodic inhibitors, The SEM analysis showed that theses inhibitors acts by establishment of a thin film at the metal surface. The film, act as a barrier to the transport of the metal ions from the metal to the solution at high concentration of inhibitor.
Keywords
Corrosion; Inhibition; Heterocyclic Compounds; Iron Phosphoric Media.
Introduction
The use of inhibitors is an important method of protecting materials against deterioration from corrosion [1-3]. Review including extensive listing of various types of organic inhibitors has been published [4]. Compound with functional group containing heteroatom which can donate lone pair electrons are found to be particularly useful as inhibitor for corrosion metals. The corrosion inhibition of iron and its alloys by different inhibitors in acidic medium has been studied by several authors [5-9]. Organic heterocyclic compounds containing azole nucleus have been found to be effective inhibitors in acidic for steel in different corrosive media [10-15]. The corrosion-inhibiting property of these compounds is attributable to their molecular structure. The planarity (Π) and the numbers of lone pairs of electrons present on heteroatoms are the important structural features that determine adsorption on the metal surface. For example Bentiss et al and Lagrenée et al [16,17] have studied the effect of addition of triazole and their derivatives on the corrosion of mild steel in acidic 1M HCL and 0.5M H2SO4. They found a high inhibition efficiency value allotted to the electronic density around pyrazolic nitrogen of the cycle [18]. Bellaouchou et al. [19-25] showed that, the inhibitive efficiency of Benzotriazole (BTAH) for 904L stainless steel in phosphoric acid, this investigation reveals that BTAH is an effective inhibitor and the inhibition efficiency increase with increasing concentration of this inhibitor.
In the present investigation, the corrosion inhibiting behaviour of 2-pyrone, a new class of heterocyclic compounds, was investigated on stainless steel in acidic solutions. Compounds HPU1 and HPU2 were prepared from one-pot three-component condensation reaction of 4-hydroxy-6-methyl-2-pyrone, urea and aliphatic aldehydes under acidic conditions in methanol (Fig. 1).
The structures of compounds HPU1 and HPU2 were confirmed by using IR, 1H NMR, 13C NMR and MS: [(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-phenyl-methyl]-urea (HPU1) IR (KBr): ν 3397, 3350, 3312, 1696, 1640 cm-1. 1H NMR (DMSO-d6, 300 MHz): δ 2.15 (s, 3H, CH3), 5.85 (s, 1H, NH2), 6.07 (s, 1H, =CH), 6.19 (d, 1H, J = 9.7 Hz, CH), 6.79 (d, 1H, J = 9.6 Hz, NH), 7.15-7.23 (m, 5H, HAr), 11.79 (s, 1H, OH). 13C NMR (DMSO-d6, 75 MHz): δ 19.4 (CH3), 46.1 (CH), 99.9 (=CH), 102.9 (C), 125.8 (2CH), 126.2 (CH), 127.8 (2CH), 143.4 (C), 158.1(CONH2), 161.6, 163.8 (2C), 165.3 (CO ester). MS (ESI): m/z 275 (M+1)+.
[(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-(4-methoxy-phenyl)-methyl]-urea (HPU2)
IR (KBr): ν 3404, 3354, 3317, 1697, 1650 cm-1. 1H NMR (DMSO-d6, 300 MHz): δ 2.10 (s, 3H, CH3), 3.64 (s, 3H, OCH3), 5.97 (s, 1H, NH2), 6.01 (s, 1H, =CH), 6.12 (d, 1H, J = 9.7 Hz, CH), 6.75 (d, 2H, J = 8.5 Hz, HAr), 7.12 (d, 2H, J = 8.5 Hz, HAr), 7.30 (d, 1H, J = 9.7 Hz, NH) 11.58 (s, 1H, OH). 13C NMR (DMSO-d6, 75 MHz): δ 19.3 (CH3), 46.1 (CH), 55.0 (OCH3), 99.9 (=CH), 103.5 (C), 113.3 (2CH), 126.9 (2CH), 135.3, 157.7, 158.2, 161.3, 163.5 (5C), 164.9 (CO ester). MS (ESI) : m/z 335 (M+1)+.
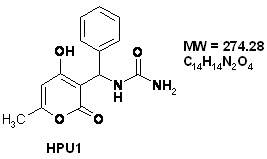
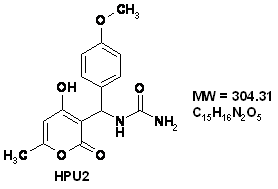
Figure 1. The chemical formulae of used inhibitors
Experimental
The electrodes were made of commercial 316 stainless steel specimens of the chemical composition (in percentage) (Table 1). For electrochemical measurements wires with 1 cm2 surface were. The steel surface was mechanically polished with emery paper, cleaned with distilled water and degreased in ethanol, washed by distilled water and finally dried in air. The polarisation studies were carried out in 30% phosphoric acid solution polluted by Cl- and SO42- potentiodynamically using a Voltalab PGZ 100 (Radiometer) controlled by a computer and employing a three electrodes cell assembly. A saturated calomel electrode (SCE) and platinum electrode were used as reference and auxiliary electrodes respectively. All solutions were prepared from analar grade chemical and bidistilled water. The experiments were performed at 25-60°C using an ultrathermostatic bath. Electrochemical impedance spectroscopy (EIS) was carried out at Ecorr after 30min of immersion in solution. After the determination of steady- state current at a given potential. Sine voltage (10mV) peak to peak, at frequencies between 100 KHz and 10 MHz was superimposed on the rest potential. The impedance diagrams are given in the Nyquist representation.
The organic inhibitors used in this work were [(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-phenyl-methyl]-urea (HPU1) and [(4-Hydroxy-6-methyl-2-oxo-2H-pyran-3-yl)-(4-methoxy-phenyl)-methyl]-urea (HPU2) (Fig.1).
Table 1. Chemical composition (in percentage) of commercial 316 stainless steel
|
specimens |
Chemical Composition |
||||||||||
|
316 |
C |
Si |
Mn |
Cr |
Ni |
Mo |
Cu |
N |
P |
S |
Fe |
|
0.02 |
0.50 |
1.71 |
16.2 |
11 |
2.18 |
0.35 |
0.07 |
0.03 |
0.023 |
Bal |
|
Results and Discussion
Open-circuit potential
Figure 2 shows the variation of free-potential with time of steel in phosphoric acid with and without inhibitors, In the case of Blank solution the electrode potential tend to decrease and stabilise after 20min at (-700mv), in the addition of inhibitors the open-circuit potential, stabilized at values between (-500mV) in the presence of (HPU2) and (-450mV) in the case of inhibitor (HPU1).
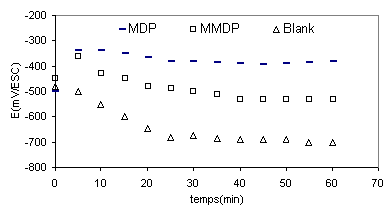
Figure 2. Open-circuit potential of the stainless steel electrode in phosphoric acid with and without inhibitors
Polarisation measurements
Figure 3 shows the cathodic and anodic polarisation curves of steel in polluted phosphoric acid {30%H3PO4 + 330ppm SO42- + 1000ppm Cl-} with and without addition of (HPU1) and (HPU2), Table 2 gives the values of the associated electrochemical parameters. The corrosion inhibition efficiency (E %) was calculated by:
E% = [(Icorr Icorr) / Icorr] × 100 (1)
were Icorr and Icorr are the uninhibited and inhibited corrosion current densities, respectively, determined by extrapolation of the cathodic and anodic Tafel lines to corrosion potential (Ecorr). As can be seen from Figure 3, cathodic current-potential curves give rise to parallel Tafel lines indicating that the hydrogen evolution reaction is activation controlled and the addition of the inhibitors studied does not modify the mechanism of this process. The value of the corrosion potentials (Ecorr) is modified by the addition of pyrimidic compounds. The addition of the compounds studied decreases the current densities in a large domain of cathodic potentials. This result indicates that theses compounds acts as cathodic inhibitors. The decrease in Icorr was pronounced with (HPU2) (Table 2). The inhibition efficiency (E%) of (HPU2) is higher than the (HPU1) this behaviour can be attributed to the presence of more electron donor groups (4 O, N, aromatic cycle) in the molecular structure of HPU2. The presence of more free electron pairs favours the adsorption of HPU2.
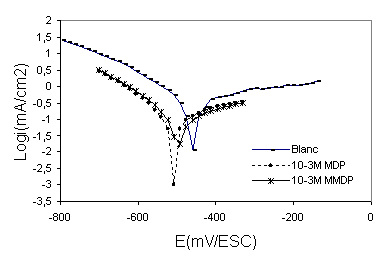
Figure 3. Polarisation curves of 316 stainless steel in phosphoric acid without and with 10-3M of inhibitors HPU2 and HPU1
Table 2. Electrochemical parameters for stainless steel type 316 in phosphoric acid in presence of inhibitors HPU2 and HPU1
|
Compound(M) |
R1(Ω.cm2) |
R2(KΩ.cm2) |
C(μF.cm-2) |
E% |
|
Blank |
3.230 |
1.790 |
63.020 |
|
|
10-3 M HPU1 |
1.400 |
6.007 |
94.300 |
70.2000 |
|
10-3 M HPU2 |
1.030 |
8.960 |
15.000 |
80.000 |
EIS measurements
The corrosion behaviour of steel, in phosphoric acid solution, in the absence and presence of inhibitors, is also investigated by EIS ambient temperature after 30min of immersion (Fig 4). The locus of Nyquist plots is regarded as one part of semi circle. The same result as obtained with Kraljic in phosphoric acid [20]. The equivalent circuit model employed for this system is shown in Figure 5. It is represented by the solution resistance (Rs), the charge transfer resistance Rtc and the double capacitance at HPU2/ solution interface. The charge-transfer resistance (Rtc) value is calculated from the difference impedance at lower and higher frequencies. The double layer capacitance (Cdl) and the frequency at which the imaginary component of the impedance is maximal (-Zmax) are determined. The inhibition efficiency got from the charge transfer resistance is calculated by:
E(%) = 100 × (Rt / inh Rt) / Rt / inh (2)
Rt and Rt / inh are the charge transfer resistance values without and with inhibitor, respectively. The impedance parameters derived from these investigations are summarised in Table 3. The inhibition efficiency, as determined from EIS methods, was found to vary in order HPU1 < HPU2 which is in a good agreement with the result obtained by potentiodynamic measurement. This result is mainly due to the polarity of molecule. It is well known that different substituents on the organic molecule polarize the functional group in a different manner [21]. The presence of OCH3 in HPU2 may increase the polarity and absorbability of inhibitors on the surface of alloy.
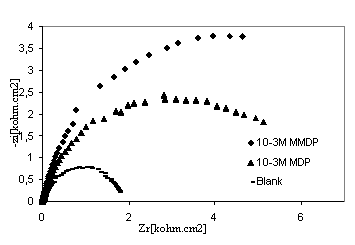
Figure 4. Nyquist diagrams of 316 stainless steel in phosphoric acid with and without HPU2 and HPU1 inhibitors
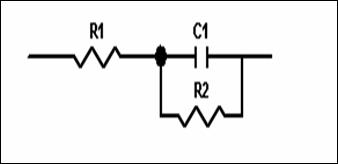
Figure 5. The equivalent circuit of used in the modelling of the electrochemical impedance spectroscopy data
Table 3. Impedance parameters for stainless steel type 316 in phosphoric acid in presence of inhibitors HPU2 and HPU1
|
Compound(M) |
R1(Ω.cm2) |
R2(KΩ.cm2) |
C(μF.cm-2) |
E% |
|
Blank |
3.230 |
1.790 |
63.020 |
|
|
10-3 M HPU1 |
1.400 |
6.007 |
94.300 |
70.200 |
|
10-3 M HPU2 |
1.030 |
8.960 |
159.000 |
80.000 |
Detailed study of HPU2
Polarization measurements
The polarisation curves of steel in polluted H3PO4 with different concentrations (10-7-10-3M) of HPU2 at ambient temperature are represented in Figure 6. It is clear that the inhibition increases as the HPU2 concentration increases. The Tafel lines slightly the same, indicate that the mechanism of the hydrogen evolution reaction (HER) not changes. As the concentration increase the polarisation for (HER) increase at a specific current, there is no significant difference in polarization between the concentration 10-4 and 10-3 M of HPU2, this corresponds to a concentration near the critical micelle concentration for the HPU2 surfactant. For the anodic branches, the shift in the anodic line is more significant in less concentration.
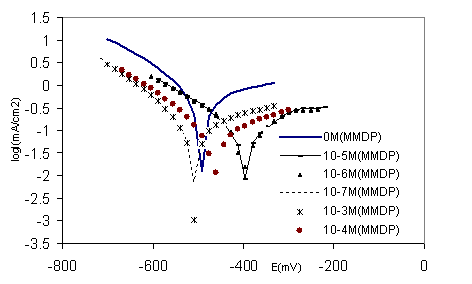
Figure 6. Polarisation curves of 316 stainless steel in phosphoric acid with HPU2 inhibitor at different concentrations
Values of associated electrochemical parameters and corrosion inhibition efficiency as function of HPU2 concentration are given in Table 4. From this table and Figure 6 it can be concluded that the presence of HPU2 at less concentration decease the anodic current, and caused a shift of the potential to more positive values making emphatic the anodic inhibitor behaviour of the HPU2, for the higher concentration of HPU2 (10-4 and 10-3 M) it is noticed that slightly more negative potentials are reached and the current density decrease more in cathodic branche compared to these obtained with low concentration. It indicates that the HPU2 is catodic inhibitor at higher concentration.
E% increases with inhibitor concentration to reach a maximum value at 10-3M HPU2.
The addition of HPU2 does not change the mechanism of the reduction process.
Table 4. Electrochemical parameters for stainless steel type 316 in phosphoric acid in presence of different concentrations of inhibitor HPU2
|
Compound (M) |
Ecorr (mV) |
Icorr (mA.cm-2) |
Rp (Ωcm2) |
-βc (mV) |
Βa (mV) |
E% |
|
Blank |
-446 |
0.76 |
123.4 |
-220 |
481.4 |
|
|
10-7 M HPU2 |
-433 |
0.18 |
191 |
164 |
149 |
76% |
|
10-6 M HPU2 |
-428 |
0.15 |
199 |
176 |
185 |
80% |
|
10-5 M HPU2 |
-438 |
0.087 |
378 |
167 |
220 |
88.50% |
|
10-4 M HPU2 |
-472 |
0.08 |
418 |
157 |
408 |
89% |
|
10-3 M HPU2 |
-472 |
0.078 |
417.78 |
150.8 |
417.8 |
90% |
Impedance measurement
Figure 7 shows the behaviour of stainless steel in acidic solution in the presence of different concentrations of HPU2, as mentioned above, Nyquist plots are not perfect semicircles. This feature had been attributed to frequency dispersion [22]. Impedance parameters derived from this investigation are given in Table 5. As the HPU2 concentration increase, Rtc values increased, but Cdl values tended to decease. The decease in Cdl values was caused by adsorption of HPU2 and decrease in local dielectric constant and /or an increase in the thickness of the electrical double layer. Suggested that HPU2, function by adsorption at the metal-solution interface [23].
The EIS measurements were also carried out at values of steel corrosion potential at different time of immersion as indicated in Figures. 8 and 9. At that potential Stainless steel- HPU2 electrode exhibit higher value of Rtc, than the steel electrode, it imposes certain barrier to the corrosion processes by forming a layer. By the increase of the layer thickness, Rtc also increases. However after a certain period of time (2h), Rtc decease, this behaviour may be due to the damaged of the layer caused by hydrogen evolution.
At high immersion time (3h), the circle is semi-circle present an inductive loop at higher frequency, the presence of inductive loop is attributed to the relaxation of intermediate reactions the similar slight is obtained with steel in H2SO4 in presence of diamine compound [24]. The relationship between Cdl with immersion time for mild steel in phosphoric acid, without inhibitor (blank), deceased with immersion time as shown in Figure 10. The same trend was observed for polluted phosphoric acid with inhibitor.
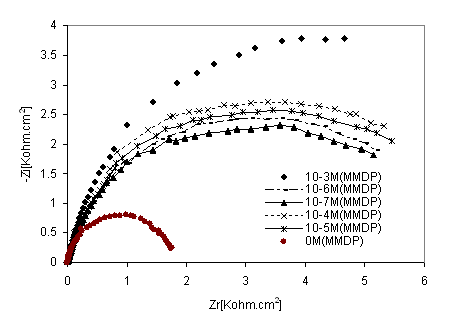
Figure 7. Nyquist diagrams of316 stainless steel in phosphoric acid with HPU2 inhibitor at different concentrations
Table 5. Impedance parameters for stainless steel type 316 in phosphoric acid in presence different concentrations of inhibitors HPU2
|
Compound(M) |
R1(Ω.cm2) |
R2(KΩ.cm2) |
C(μF.cm-2) |
|
Blank |
3.23 |
1.79 |
63.02 |
|
10-7 M HPU1 |
1.66 |
6.15 |
92.04 |
|
10-6 M HPU1 |
1.14 |
6.4 |
88.41 |
|
10-5 M HPU1 |
1.16 |
6.8 |
92.6 |
|
10-4 M HPU1 |
1.47 |
6.86 |
93.5 |
|
10-3 M HPU2 |
1.03 |
8.96 |
159 |
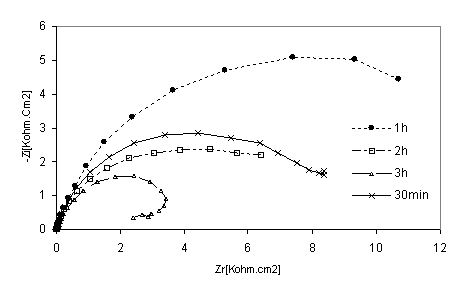
Figure 8. Nyquist diagrams of 316 stainless steel in phosphoric acid with HPU2inhibitor at different immersion time
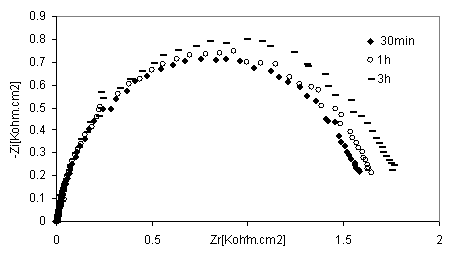
Figure 9. Nyquist diagrams of 316 stainless steel in phosphoric acid with without inhibitor at different immersion time
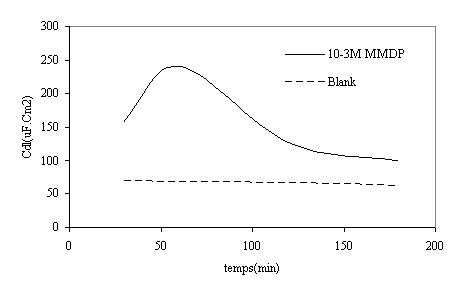
Figure 9. the variation of Cdl with immersion time in presence and absence of HPU2 in polluted phosphoric acid.
Conclusion
Weight loss measurements, polarization curves and AC impedance measurements were used to investigate the effect of the inhibitor on the organic inhibitors on the corrosion of stainless steel in phosphoric acid. The presence of the organic inhibitors in the corrosive medium decreases the corrosion current (Icorr). The inhibition efficients increases with inhibitors concentrations, reaching a maximum at 10-3M. Steady state measurements show that both compounds do not change the hydrogen reduction mechanism. The best performance was obtained with HPU2 inhibitor.
References
1. Bregmann J. I., Corrosion inhibitors, P. T. Macmillan, New York, 1963.
2. Hackerman N., Comprehensive Treatise of Electrochemistry, Langmuir, 1987, 3, 922-930.
3. Nathan C. C., Organic inhibitors, Nace, Houston, 1977.
4. Trabanelli G., Caraiti V., in: M.G.Fontana, R.W. Staehle (Eds.) Advances in corrosion science and technology, Vol., Plenum Press, NY, 1970, pp.147.
5. Vosta J., Pelikanj S. M., Practical Aspects of Corrosion, Werkst. Korros, 1974, 25, p. 750-756.
6. Sathiyanarayanan S., Dhawan S. K., Trivedi D. C., Balakrishanan K., Prevention of corrosion of iron in acidic media using poly (o-methoxyl-aniline), Corros. Sci., 1992, 33, 1831-1840.
7. Zvauya R., Dawson J. L., Electrochemical reduction of carbon dioxide and the effect of the enzyme carbonic anhydrase 11 on iron corrosion, J. Appl. Electrochem. 1994, 24, p. 943-952.
8. Mohamed A. K., Abdel-Maksoud S. A., Fouda A. S., 2-Hydroxyacetophenone-aroyl Hydrazine Derivative as Corrosion Inhibitors for Copper Dissoulution in Nitric Acid Solution, Bull. Soc. Chim. Beg. 1996, 105, p. 363-370.
9. Aksut A. A., Onal A. N., Corrosion Inhibitionof Aluminium Alloysby Toliltreazole in Chloride Solution, Corros.Sci., 1997, 39, p. 76-86.
10. Abdenaly B. A., Eltothy A., Elgamal M., Mahgoub F., Deposition, properties and applications of PVD CrxN coatings, Surf. Coat. Technol., 1986, 27, p. 325-330.
11. Tadrog A. B., Abdenaby B. A., Immobilization of Metalloporpherine in Electropolymerized Films, Acc. Chem. Res., 1995, 28, p. 30-36.
12. F. Zucchi, V. Grassi, C. Monticelli and G. Trabanelli, Hydrogen embrittlement of duplex stainless steel under cathodic protection in acidic artificial sea water in the presence of sulphide ions, Corros. Sci., 2006, 48, p. 522-530.
13. Bekkouch K., Aouniti A., Hammouti B., Kertit S., Substituted uracils as corrosion inhibitors for copper in 3% NaCl solution, Corrosion Science., 2003, 45, p. 1619-1630.
14. Kertit S., ES-Soufi H., Hammouti B., Benkaddour M., 1-phenyl-5-mercapto-1,2,3,4 tetrazole (PMT):un nouvel inhibiteur de corrosion de lalliage Cu-Znefficace à très faible concentration, J. Chim. Phys., 1998, 95, p. 2070-2082.
15. Refaey S. A. M., Taha F., Abd El-Malak A. M., Inhibition of stainless steel pitting corrosion in acidic medium by 2-mercaptobenzoxazole, Appl. Surf. Sci., 2004, 236, p. 175-185.
16. Bentiss F., Lagrenée M., Traisnel M., Hornez J. C., Corrosion inhibition of mild steel by the new class of inhibitors [2, 5-bis(n-pyridyl)-1, 2, 4-thiadiazoles]in acidic media, Corros. Sci., 2001, 43, p. 2229-2238.
17. Bentiss F., Traisnel M., Lagrenée M., The substituted 1, 3, 4-oxadiazoles: a new class of corrosion inhibitions of mild steel in acidic media, Corros. Sci., 2000, 42, p. 703-719.
18. F. Bentiss, M. Traisnel, M. Lagrenée, The inhibition action of 3, 6-bis(2-methoxyphenyl)-1, 2-dihydro-1, 2, 4, 5-tetrazine on the corrosion of mild steel in acidic mediaCorros. Sci. 42 (2000) 127-146.
19. Bellaouchou A., Kabkab B., Guenbour A., Ben Bachir A., Corrosion inhibition under heat transfer of 904L stainless steel in phosphoric acid by benzotriazole, Proc. Org. coat., 2001, 41, p. 121-127.
20. Kraljic M., Mandic Z., Duic L. J., Inhibition of Steel Corrosion by Polyaniline Caoting, Corros. Sci., 2003, 45, p. 181-198.
21. Kertit S., Bekkouch K., Hammouti B., Inhibition de la corrosion d'un acier au carbone en milieu H3PO4 2M par des composés organiques de type tetrazole = Corrosion inhibition of a carbon steel in 2M H3PO4 medium by tetrazole : type organic compounds, Rev. Metal. Paris, 1998, 95, p. 251-257.
22. Mansfeld F., Kendig M. W., Tsai S., Recording and analysis of AC impedance data for corrosion studies II. Experimental approach and results, Corrosion, 1982, 38, p. 570-580.
23. Bentiss F., Lagrenée M., Traisnel M. And L. Gengembre, Inhibition of acidic corrosion of mild steel by 3, 5-diphenyl-4H-1, 2, 4-Triazole, Applied Surface, 2000, 161, p. 194-202.
24. Ouchrif A., et al., 2, 3-Quinoxalinedione as a novel corrosion inhibitor of mild steel in 1M HCl, Materials Chemistry and Physics, 2007, 105, p. 1-5.
25. Hnini K., Chtaini A., Inhibition of metallic corrosion with eugenol, Bulletin of Electrochemistry, 2004, 20, p. 481-485.