Evaluation and Treatment of Coal Fly Ash for Adsorption Application
Samson Oluwaseyi BADA* and Sanja POTGIETER-VERMAAK
School of Chemical and Metallurgical Engineering, Faculty of Engineering and the Built Environment, University of the Witwatersrand, P/Bag X3 Wits 2050 Johannesburg, South Africa.
E-mail: Samson.Bada@students.wits.ac.za
Abstract
Many researchers had investigated fly ash as an adsorbent for the uptake of organic compounds from petrochemical waste effluents. The availability, inexpensive and its adsorption characteristic had made it an alternative media for the removal of organic compounds from aqueous solution. The physical property of South African Coal Fly Ash (SACFA) was investigated to determine its adsorption capability and how it can be improved. Chemical treatment using 1M HCl solution in the ratio of (1 g) fly ash to (2 ml) of acid was used and compared with untreated heat-treated samples. The chemically treated fly ash has a higher specific surface area of 5.4116 m2/g than the heat-treated fly ash with 2.9969 m2/g. More attention had to be given to the utilization of SACFA for the treatment of wastewaters containing organic compounds through the application of Liquid phase adsorption process that was considered as an inexpensive and environmentally friendly technology.
Keywords
Fly ash; Adsorption capacity; Adsorbent; Characterization.
Introduction
Coal firing power thermal stations are still the main source of power generation in South Africa and these stations are situated in close vicinity of the coalfields, all in the North of the Country (Gauteng, Mpumalanga, Limpopo and Free State). Sasol, which is one of the Africas major producers of chemicals and liquid fuels and Eskom, a major power utility in South Africa are some of the biggest consumers of coal in South Africa with Sasol utilizing 28 million tons of coal for its gasification process at Sasol synfuels in Secunda and 6 million tons at Sasol Infrachem for Sasolburg. These plants generated 7 million tons of gasification ash and 1.5 million tons of ash respectively in 2005 [1].
South Africa currently produces more than 25 million tons of ash per annum, of which nearly 1.2 million tons are utilized for different purposes [2], i.e as back mine fill, as soil stabilizer in geotechnical application, like wise in land fill, also as an extender and pozzolan for cement and concrete applications, and as adsorbent for inorganic wastes. Previous research conducted on South African fly ashes includes, the investigation on the surface properties of an ultra fine fly ash regarding its contact and interaction in the polymer industry [3]. The use of fly ash in cement by [4] and [5] also investigated the extraction of alumina from low rank class F South African coal. While, [6] had successfully utilized South Africa fly ash for the removal of phosphate from aqueous solution. The authors are not aware of any open literature on investigating the method of improving the adsorption capability of South African fly ash.
Currently, researchers are now being focused on how to improve the capability of fly ash through proper beneficiation techniques in order to increase its adsorption rate. A Sonochemical technique with NaoH was applied by [7] for the treatment of fly ash in order to increase its specific surface area and its adsorption capacity. Surface modification technology involving addition of HCl to improve the surface morphology and specific surface area of fly ash had been reported [8].The steam activation of unburned carbon in fly ash was investigated in order to promote the development of micropores which generated an activated carbon with surface areas of 825m2/g [9].
The importance of beneficiation in the utilization of fly ash for a particular application cannot be neglected. Beneficiation techniques are used to influence the characteristic of fly ash in order to optimize its utilization, increase its value and minimize disposal cost. These techniques aid researchers in investigating the properties of fly ash and how it can be improved to produce a quality controlled fly ash product for removal of inorganic and organic compounds and also for other higher value applications in the polymer and ceramic industries [10]. The knowledge of fly ash mineralogy, the degree of the unburned carbon in fly ash and the quality needed in the market-place are supreme in creating an opportunities for research into the modification and exploitation of the unique chemistry of fly ash [14]. Therefore, the study on the importance of beneficiation of fly ash had lead to comprehensive information regarding the feasibility of fly ash for adsorption process. It has been confirmed that the utilization of fly ash would solve both disposal problem and served as a cheaper material for adsorption of water pollutants. The chemical characteristic of fly ash which depends strongly on the geological origin of the coal, method and condition of combustion would be one of the important factors to be considered [12]. Adsorption, which is a surface phenomenon, that depends on the higher specific surface area, narrow particle size distribution and the porosity of an adsorbent were also investigated for this fly ash. [13] observed that the larger the specific surface area, the higher the carbon content and the finer the particle size of the fly ash the greater its adsorption capacity will be. A large amount of information is now available to improve the adsorption capability of fly ash such as the chemical treatment method conducted by [14].
The adsorption capability of the South African Coal Fly Ash (SACFA) has been investigated, with the main objective of evaluating the property of fly ash when subjected to chemical treatment and heat treatment. In addition, to predict if SACFA can be use for the removal of organic and inorganic compounds from wastewaters considering its physical properties as compared to those in the literature
Material and Method
Convectional chemical and heat- treatment
The low grade pulverised coal fly ash was obtained from the Sasol plant in South Africa. Sedimentation process was implemented after the mixture of the fly ash (FA) (500 g) with double- deionised water (1000 ml) to remove the soluble inorganic matter that are present in order to eliminate pores clogging. Hence, the two samples were prepared from the fly ash (FA). The FA was divided into two and one sample referred to as heat-treated fly ash (HFA) was obtained by heat treating it for 12h in a convectional oven at 105ºC and allow to furnace cool. The chemical heat-treated fly ash (AHFA) was obtained after being subjected to chemical treatment. The sample was mixed in 1M HCl solution in the ratio of (1g) fly ash to (2ml) of acid, filtered and heat treated at 105ºC for 12 h in a convectional oven and also allow to furnace. After treatment both samples were stored inside different desiccators.
Characterization techniques
The specific surface area of the fly ash was obtained using a TriStat 3000 analyzer (Micromeritics Instrument Corp) with N2 adsorption at - 196ºC. The sample was first degassed at 200ºC for 4 h. TriStat 3000 is an automated gas analyzer which contains three ports, allowing up to three samples to be analyze simultaneously. The TriStat 3000 system consists of the TriStar analyzer, a SmartPrep degasser that is used for samples preparation, a vacuum pump and a control module for entering analysis and report options.
The mineralogical composition of the fly ash was determined by the X-ray diffraction analyses for the qualitative evaluation of the common and predominant phases within the ash. The phase identification of the representative samples of the fly ash that is in a powdery form, were determined by the X-ray diffractometry using a Phillips PW 1830 diffractometer with a Cu-anode. The diffractometer was operated at 40 kV and 40 mA for 1 h over the range of 2q from 0° to 80° and the identification was carried out with High Score Plus software.
The Scanning Electron Microscopy (SEM) model Jeol JSM840 was used to determine the morphological and qualitative characteristics of the ash. The SEM was operated at the accelerating voltage of 20 keV for mineral analysis of representative samples in order to provide information on the physical properties of the ashes. The fly ash sample was carbon coated in order to make its surface conductive.
The particle size of the fly ashes was measured using a laser based particle size analyzer, namely a Mastersizer 2000 of Malvern Instruments Ltd. It utilizes Fraunhofer diffraction of light formed by particles with a diameter larger than the incident laser beam wavelength. A combination of an optical filter, lens and photo detector coupled with a computer loaded with Mastersizer software enables one to compute the particle size distribution from the diffraction data and store it as volume percentage against the particle size.
Results and Discussion
XRD analysis
The diffractogram (Figure 1) shows the X-ray diffraction pattern for particle size ≤ 30 μm. It was found that the fly ash consisted of crystalline minerals mullite, quartz, hematite and small amounts of calcium oxide with large characteristic peaks of quartz (SiO2). This result is similar to that reported for a fly ash investigated by [15]. The intensity of quartz is very strong, with mullite forming a chemically stable and dense glassy surface layer. The low calcium oxide intensity is characteristic of low-Ca Class-F CFA, and similar to the result reported by [16].
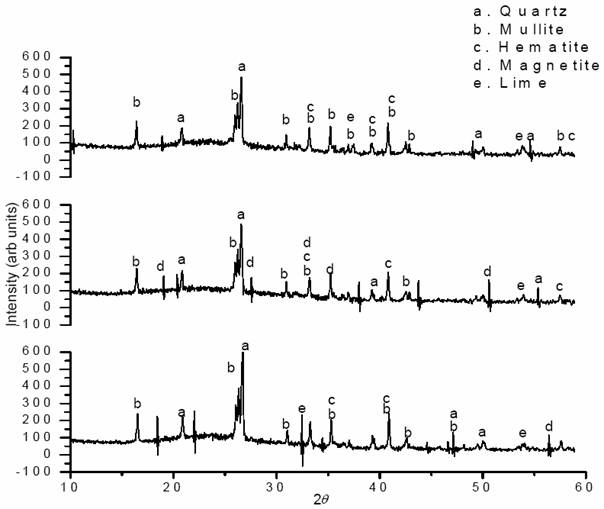
Figure 1. X-ray
diffraction pattern of fly ash. (a)As-received fly ash; (b) Heat treated fly
ash; (c) Acid treated fly ash
It can be observed that there are no significant differences for all XRD profiles but the large characteristic peaks of quartz (SiO2) in the AHFA than those for HFA and FA are indicative of large SiO2 concentrations. The As-received fly ash (FA) shows the existence of mullite, quartz, hematite and small amounts of lime and sodium oxide. The intensity of quartz is very strong, with mullite forming a chemically stable and dense layer. The FA is of lower activity and at this state its glassy surface layer particles is dense, chemically stable and also protected the more active inner constituents of the fly ash comprises of porous, spongy and amorphous particles. The stability of the FA can only be disintegrate to encourage chemical activity if the glassy chain of the FA which comprises of Si, low carbon and Al are subjected to chemical reaction. The reaction of HCl with fly ash rapidly disintegrates the stable glassy layer (macropore) protecting the active inner core of the ash and these glassy layers that are made up of Si-Al chain of high Si, Al and low Ca content however, corrodes. The corrosion of this outer layer leads to a chemical reaction that cracked the inner constituents of the fly ash thereby increasing the mircopore volume (fig. 8). The characteristic peak intensity of the quartz is more apparent in (fig.1) that is, quartz is more concentrated in this fraction. (Sarkar et al, 2006) observed that the increase in quartz content leads to decrease in particle size distribution of fly ash. [17] also observed that the acid treatment of fly ash will induce surface changes in the surface properties of FA and influenced the degree of adsorption of these adsorbates onto the FA surface. Considering the HFA, the formation of magnetite is more pronounced in this pattern than the other samples. The clusters of (Fe-oxide) particles in (fig.5) through the heat- treatment may provide the basic cation for nucleation of some hematite particles to form magnetite microspheres seen in (fig. 6 & 7).The major phases for the entire fractions are quartz and mullite, with the existing of hematite and magnetite as minor phases. Therefore, in this result acid treatment will not induce changes in bulk phase.
Specific surface analysis
The specific surface areas (SBET) for the two fly ashes used in this analysis are presented in Table 1. This result confirms that fly ash treated with 1M of HCl solution in a convectional oven at the temperature of 105ºC for 12h has a higher surface area of 5.4116 m2 /g, while the untreated fly ash heat treated at 105ºC for 12h exhibited a lower SSA (SBET) of 2.9969 m2 /g.
Table 1. Specific Surface Area of the Fly Ash
|
Sample |
SBET (m2 /g) |
|
HFA |
2.9969 |
|
AHFA |
5.4116 |
Particle size distribution
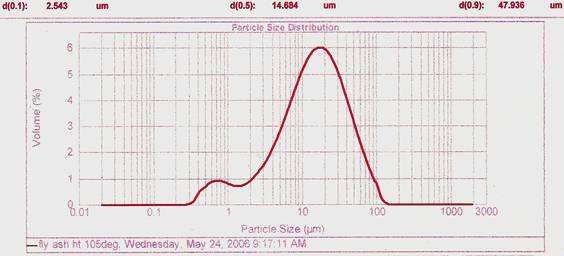
Figure 2. Particle size distribution profile for Fly Ash Heat treated at 105°C
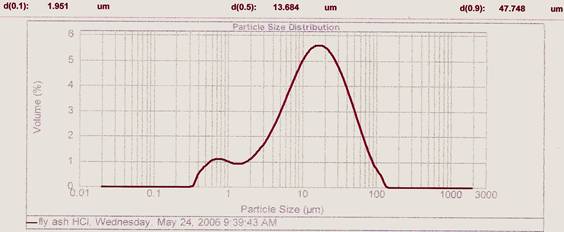
Figure 3. Particle size distribution profile for Acid treated Fly Ash in 1M HCl.
The grain size distributions of these two fly ashes (fig. 2 & fig. 3) can be grouped as Normal - Gaussian particle size distribution with modes in the region of 10-30 µm. The AHFA had a smaller particle size than that of the HFA. [18] observed that for effective adsorption small particle sizes and large surface areas are required for high adsorbate removal at equilibrium. [19] confirmed that the larger the specific surface area and the finer the particle size distribution of adsorbent, the greater its adsorption capacity and interaction with an adsorbate The two fly ashes have higher population densities at < 20 and < 30 µm which may be an added advantage for there utilization in the construction sector.
SEM analysis
|
|
|
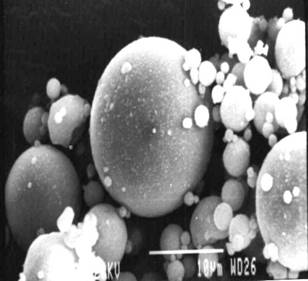
Figure 4 & 5. SEM micrographs of Fly Ash
|
|
|
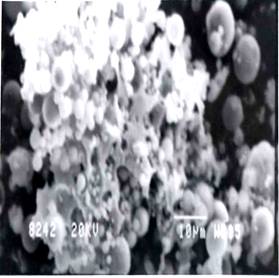
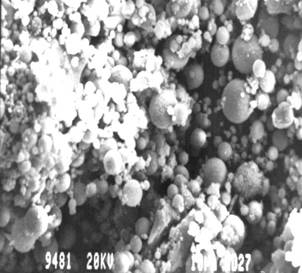
Figure 6 & 7. SEM micrographs of heat - treated Fly Ash at 105°C
|
|
|
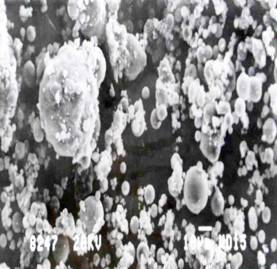
Figure 8 & 9. Micrographs of Acid heat-treated fly ash at 105°C
The investigation reveals that most of the particles present in the fly ash are spherical in shape with a relatively smooth surface grain. Fig.4 shows sub-angular and spherical particles with relatively smooth grains consisting of quartz, while fig. 5 shows clusters of iron (Fe-0xide) particles formed due to partial decomposition of pyrite and with dark quartz inclusions. Similar results were obtained from the investigation conducted by [1] on Sasol ashes. The HFA, (fig. 6 & 7) shows a decrease in the particle size as compared to FA sample. The decrease might be because of the increase in the spheriodal nature of the magnetic particles formed from the transformation of some hematite phases into magnetite (fig.2). Fig.8 and 9, show a marked segregation of oblong spherical particle of Fe-oxide but with a large portion of both fractions consists of spheres. A crack is also generated in the matrix of glass which maybe responsible for an increase in adsorbent pore volume.
Conclusions
The application of fly ash for the treatment of organic waste effluents is gaining attention as a cost effective, simple and environmentally save means of wastewater treatment. Fly ash is an adsorbent that is inexpensive and readily available in South Africa. In addition, FA possesses an effective characteristic that make it a media for an organic compounds uptake but its adsorption capability can be improved on by using convectional chemical heat treatment. The characterization of fly ashes was conducted to determine the changes impacted on its physical properties due in order to compare their possible adsorption capability when used as an adsorbent in treating organic waste effluents.
- Acid treatment induces changes in the specific surface area of the fly ash from 2.9969 m2/g to 5.4116 m2 /g. This was achieved through the corrosion of the outer layer of the fly ash to ash to disintegrate its stable glassy layer.
- The SEM result shows the crack generated by the corrosion of the outer layer of the fly ash which leads to a chemical reaction that exposed the inner constituents of the fly as thereby increasing the micropore volume.
- The HFA generally exhibits high adsorption capacity but with the HCl treatment its adsorption capability can be improved through the increase in its specific surface area and an induced changes in its surface properties.
References
1. Matjie R. H., Ginster M., Van Alphen C. and Sobiecki A., Detailed Characterization of Sasol Ashes, 2005, http://whocares.caer.uky.edu/wasp/AshSymposium/AshLibraryAgenda.asp#2005, (Cited, 19 September 2006).
2. Kruger R. A, South African Coal Ash Association, Personal Communication, 2002, Johannesburg.
3. Potgeiter-Vermaak S. S., Potgeiter J. H., Kruger R. A., Spolnik Z. and Van Grieken R., A characterisation of the surface properties of an ultra fine fly ash (EFFA) used in the polymer industry, Fuel, 82, 2005, p. 2295-2300.
4. Helmuth R., Fly ash in cement and concrete, Portland Cement Association, Skokie, 1987, p 36-61.
5. Matjie R.H., Bunt J.R. and Van Heerden., Extraction of alumina from coal fly ash generated from a selected low rank bituminous South Africa caol, Mineral Engineering, 2004, 18, p. 299-310.
6. [Agyei N. M., Potgeiter J. H. and Strydon C. A., The removal of phosphate ions from aqueous solution by fly ash, slag, ordinary cement and related blends, Cement and Concrete Research 32, 2002, p. 1889-1897.
7. Wang S., Boyjoo Y., Zhu J., Sonochemical treatment of fly ash for dye removal from wastewater, Journals of Hazardous Materials, B126, 2005, p. 91-95.
8. Sarbak Z., and Kramer-Wachowiak, Porous structure of waste fly ashes and their chemical modifications. Powder Technology, 2002, 1, p. 53-58.
9. [Schobert H. H., Maroto-Valer M. M. and Lu Zhe., Development of activated carbons from coal combustion by-products, Final technical progress report, 2003, www.osti.gov/bridge/servlets/purl/822988-POkLYx/native/822988.pdf (Cited, 17 October 2006).
10. Hwang J. Y., Unburned carbon from fly ash A hidden treasure, Institute of materials processing, conference proceeding, 1997, Michigan Technological University.
11. Kruger R. A., Kruger J. E., Historical development of coal ash utilization in South Africa, International ash utilization symposium and the world coal ash Conference, Policy 3, 2005.
12. Singh D. N. and Kolay P. K., Simulation of ash-water interaction and its influence on ash characteristic, Progress in Energy and Combustion Science, 2002, 28 (3), p. 267-299.
13. Kao P. N., Tzeng J. H. and Huang T. L., Removal of chlorophenols from aqueous solution by fly ash, Journals of Hazardous Materials, 76, 2000, p. 237-249.
14. Wang S., Boyjoo Y., Choueib A. and Zhu J., Utilization of fly ash as low cost adsorbents for dye removal, Chemeca 2004, 26-29 September, Sydney.
15. [15] Sarkar A., Bassu A.K., Udaybhanu G. and Rano R., A comprehensive characterisationof fly ash from a thermal power plant in Eastern India, Fuel, 2006, 87, p. 259-77
16. Giere R., Carleton L. E. and Lumpkin R .G., Micro- and nanochemistry of fly ash from a Coal-fired power plant, American Mineralogist, 2003, 88, p. 1853-1865.
17. Bhargava D. S. and Sheldarkar S. B., Use of TNSAC in phosphate adsorption studies and relationships. Effects of adsorption operating variables and related relationships, WaterResarch, 1993, 27(2), p. 313-324.
18. Kao P. N., Tzeng J. H., and Huang. T. L, Removal of chlorophenols from aqueous solution by fly ash. Journals of Hazardous Materials, 2000, 76, p. 237-249.