Ballonet String Model of Molecules
Gavril Niac
Department of Chemistry, Technical University of Cluj-Napoca, Cluj, Romania
E-mail: Gavril.Niac@chem.utcluj.ro
Abstract
Strings of ballonets, modelling rows of orbitals, are assembled to molecule models by crossing them properly. The ballonets at the ends of the strings of 2, 3, 4 or 5 spheres represent bonding orbitals of hydrogen with other elements like C, N or O (the proton being inside the sphere), as well as nonbonding orbitals. The ballonets between them are modelling bonding orbitals among elements other than hydrogen - except the double bond in diborane, the atomic cores laying at the junction of two or more spheres.
Advantages of elastic sphere models range from self-adjusting bond angles to resistance when closing cycles like cyclopropane or modeling double bonds.
Examples comprise alkanes, including platonic hydrocarbons, ethene, acetylene, and some inorganic molecules.
Keywords
Molecule; Structure; Ballonet string; Physical model.
Introduction
The aim of physical molecular models is to reproduce at human scale the form and behaviour of invisible and untouchable molecules. After Ramberg [[1]], physical hand-held models in chemistry are a unique mode of non-semiotic reasoning that is nevertheless highly sophisticated despite the lack of mathematics. The most important characteristics of form and behaviour are: relative bonds lengths, bond angles, space filling, rotation around single bonds and resistance towards rotation of double bonds, stress in some cycles or multiple bonds, different types of isomerism.
Most usual physical models are focused on correct bond angles and relative bond lengths (both established by project) [[2]]. Some of them allow rotation around single bonds and just a few of them give an idea about space filling [[3]]. Among these, three should be mentioned: 1) the Stewart-Briegleb model - considering atoms in molecules as spheres with cut off segments at specific angles to each other, 2) orbital models like the tangent sphere model [[4]-[10]] and 3) the balloon model of orbitals and molecules [[11]-[16]]. The last one fulfils the requirements of both form and behaviour of molecules, but in its form described in the literature is difficult to handle. A group of students at the Hamburg University [[17]] proposes molecular modelling with twist balloons. Portions of balloons represent chemical bonds like sticks; therefore, this model is like a stick model with elastic sticks.
An easy technical solution is to use inflated elongated balloons converted to strings of several ballonets, which can be assembled to molecule models [[18]].
The main advantage of such strings is simple handling, but they are also closer to reality than are other models, since atoms do not exist in molecules as spheres, like in free state. Rather the occupied orbitals with electron pairs (or single electrons) have approximately the form of spheres (or ellipsoids). On the other hand, the atomic cores are located at the junction of two or more orbitals, except bonds implying hydrogen, when the hydrogen nucleus is inside the electron cloud of the bonding pair. The ballonet string model has also the advantage that bond angles and relative bond lengths are not prefabricated, but they result automatically while assembling the model. It is also a physical version of the VESPR model of Nyholm and Gillespie [[19]-[22]], but with elastic attraction forces modelled as well.
Necessary materials
Elongated balloons, like in figure 1, and adhesive tape of size 0.2 × 1.5 cm are needed. A pump would do the inflation easier.
Construction of ballonet strings
The balloons are not fully blown up and, starting from the end, a ballonet is formed by twisting the balloon at the right place. Next, the ballonet and the rest of the balloon are pulled somewhat apart and a ~2 mm wide adhesive band is wrapped around in order to avoid airflow between the ballonet and the remnant of the balloon. Help of a second person can be welcome. The pressure in the remaining balloon is adjusted, if necessary, and the next ballonet can be formed repeating the above operations, and so on. For modelling most molecules, strings of 2 to 5 ballonets are sufficient. Balloons and strings of two (‚), four („) and five (…) ballonets are also shown in figure 1. Covering surfaces with talcum powder makes ballonets less sticky and assembling easier.
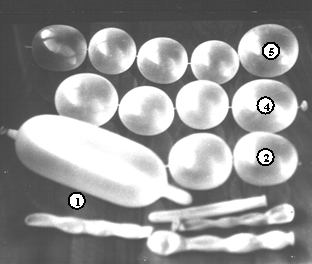
Figure 1. Some elongated balloons used to shape ballonets and some strings of ballonets
The ballonets at both ends of the strings represent electron pair clouds (orbitals) of C-H bonds, with the hydrogen nucleus inside, while the ballonets between them correspond to other bonding pairs (C-C, C-N, etc). The atomic cores of these atoms lay approximately at the junctions of ballonets so they lay outside the bonding electron cloud.
Modelling of molecules happens by superimposing two atomic cores, i.e. by twisting rows at the junction of two ballonets. Figure 2 shows how to combine the strings in order to shape some molecular models.
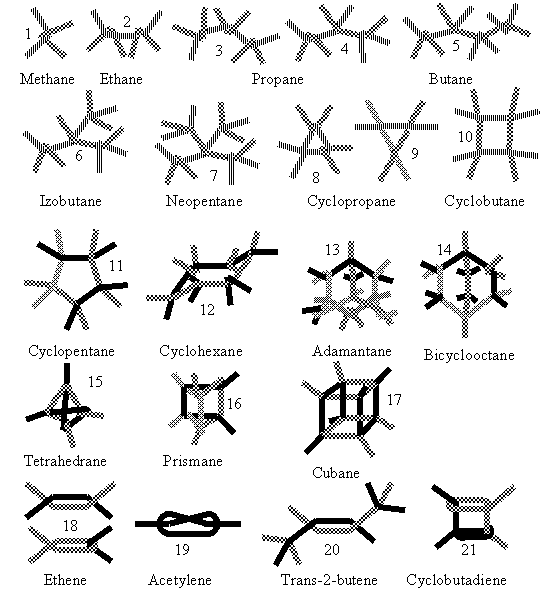
Figure 2. Assembling of some organic molecule models. Broken lines with different texture are different strings of ballonets, each line segment corresponding to a ballonet.
Examples of Ballonet Models
Normal hydrocarbons
Methane (CH4), also ammonia, water, hydrofluoric acid
The model of these molecules is the result of twisting in place, as shows figure 2, two pairs of ballonets. The model of CH4 is given in figure 3. The tetrahedral orientation of the bonding orbitals occurs automatically due to the elastic repulsion of the spheres. Water and ammonia molecule models need more dense ballonets for nonbonding electron pair cloud modelling.

Figure 3. Ballonet model of CH4. The bond angles result automatically while assembling.
In the case of NH3, H2O and FH, distinction can be made between bonding and nonbonding orbitals by inflating for the last ones larger and better filled ballonets, resulting in widening their solid angle to the centre and narrowing the H-N-H and H-O-H angles, as it happens in real molecules.
Ethane (C2H6), also methylamine, methanol, hydrazine, hydrogen peroxide
The model of ethane (and other similar molecules) can be seen in figure 4. Model can be built with one string of three and two of two ballonets, as shown in (2) figure 2.
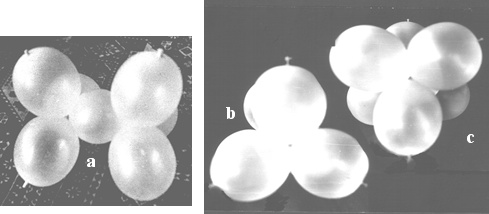
Figure 4. Ballonet model of C2H6; a. general view; b. eclipsed form; c. opposed form.
Methylamine, methanol, hydrazine and hydrogen peroxide have essentially the same shape. Refinement can be achieved, as for ammonia and water, by taking for nonbonding orbitals larger and stronger ballonets.
Propane (C3H8), also ethylamine, ethanol
Figure five shows the model of propane, which needs one string of four and three of two ballonets (or two strings of three and two of 2), as shown in figures 2.3 and 2.4.
The bend at the middle of the chain is clearly visible.
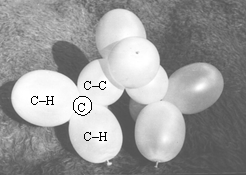
Figure 5. Model of H3C-CH2-CH3 (propane). For better understanding, some ballonets are marked: CH indicates carbon-hydrogen, while CC stands for carbon-carbon bonding orbital. Encircled C shows the place of the one of the carbon cores (at the junction of four bonding orbitals).
Butane (C4H10)
Model of the butane molecule is shown in figure 6. To build the model three strings of three ballonets each and two pairs of ballonets are needed as shown in (5) figure 2. Parts of the chains can be rotated around the single bonds, in order to visualize many of the infinite possible arrangements.

Figure 6. Ballonet model of n-butane molecule
Branched hydrocarbons
Isobutane (metylpropane, C4H10)
In figure 7 the photograph of the isobutane model is given. It was built using one string of four, one of 3 and 3 pairs of ballonets, as shown in (6) figure 2.

Figure 7. Model of the isobutane molecule
Neopentane (tetramethylmethane, C5H12)
Figure 8 shows the model of the neopentane molecule, assembled as seen in (7) figure 2.

Figure 8. Model of the neopentane molecule.
Cyclohydrocarbons
Cyclopropane, cyclobutane, cyclopentane and cyclohexane can be assembled using three, four, five and six rows of three ballonets, as shown in (8)-(12) figure 2.
Cyclopropane, C3H6
Figure 9.a. shows the model of cyclopropane before closing the ring. The ends of the row are quite far from each other, despite bringing them as close as possible by rotation around single bonds. To bring the junctions of the ballonets close to each other (in order to close the ring) an effort is needed to overcome the repulsion of the orbitals (ballonets). Figure 9.b. is the photograph of the assembled model.
A somewhat smaller effort is needed when the cyclobutane ring has to be closed, while for cyclopentane the junctions of the ballonets can be brought side by side by simple rotation around the single bonds. In the fiver ring, all junctions (carbon cores) are in the same plane. The model of cyclohexane can not have all the junctions in the same plane, so it takes either the chair, or the bath form.
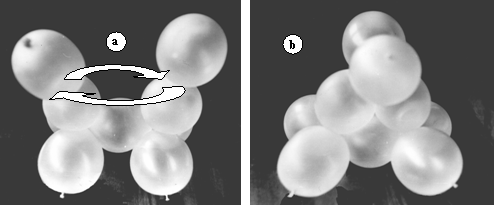
Figure 9. Model of cyclopropane; a. before twisting the ends to close the cycle; b. the ready model.
Cyclobutane, C4H8
Figure 10 shows the photograph of the cyclobutane model. This model was assembled as shown in 10 figure 2.

Figure 10. Ballonet model of the cyclopropane molecule
Carbon cores as well as C-C bonding orbitals are coplanar and situated at the corners of two different squares, rotated with 45° to each other.
Cyclopentane, C5H10
The model of the cyclopentane molecule can be seen in figure 11. Assembling is shown in 11 figure 2.
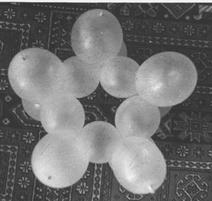
Figure 11. Model of the cyclopentane molecule
Cyclohexane, C6H12
Cyclohexan has two conformation isomeres; the chair form and the bath form. Figure 12 shows the chair form, while figure 13, the bath form. Construction mode of the chair form is given in 12 figure 2.
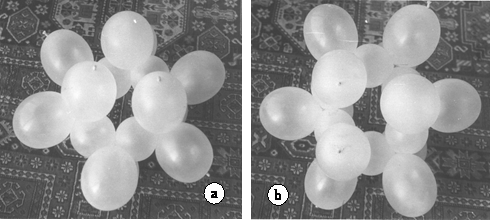
Figure. 12. Model of the chair form of cyclohexane. a. General view. b. View from above. The six radically oriented, as well as three of the axially oriented C-H orbitals, are clearly visible. The other three axially oriented orbitals are hidden, behind the molecular plane
Construction of the bath form is similar and it is easy to pas from one to the other by simple rotation of two > CH2 groups 120°.
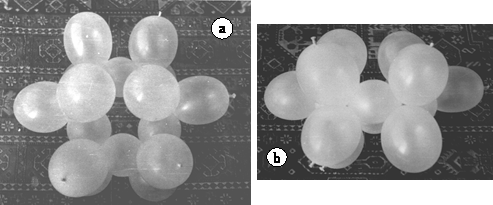
Figure 13. Model of the bath form odd cyclohexane. a. View from above. b. Lateral view
Bicyclooctane (C8H14)
The model of C8H14 needs three strings of five and four of two ballonets, as shown in (14) figure 2. It may be considered made of three condensed bath form cyclohexanes. The general view of the model can be seen in figure 14.a, while a view along the axis of the molecule (white arrows in a) is shown in figure 14.b.
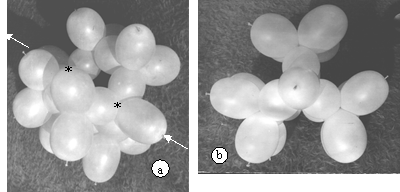
Figure 14. The bicyclooctane model. a. General view; * shows the approximate place of the two common carbon core, to the three cyclohexane rings (bath form). b. View along the molecular axis, marked in a. with arrows.
Tetrahedran, C4H4, also phosphorus, P4; cubane, C8H8
As its name suggests, C4H4 (tetrahedrane) has the carbon cores as well as the hydrogen nuclei at the peaks of tetrahedrons. A derivative of the molecule (tetra-isobuthyl-tetrahedrane) was first synthesized some 35 years ago [[23]]. To build the model 2 fiver strings of ballonets are needed. Assembling is shown in (15) figure 2. Tetrahedrane is one of the so called Platonic Molecules. Figure 15.a shows the model of tetrahedrane (as well as of P4). In the case of phosphorus, the ballonets at the four corners represent nonbonding orbitals.
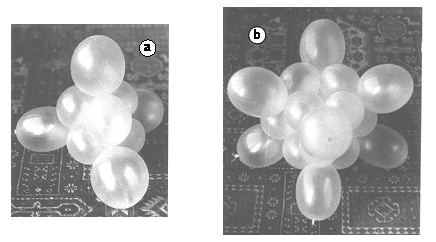
Figure 15. Models of two platonic hydrocarbons. a. Tetrahedrane. b. Cubane.
Cubane, C8H8, another Platonic Molecule, was first synthesized in 1964 [[24]] and has a surprisingly high stability, opposite to tetrahedrane. The ballonet string model can be assembled using four fiver strings, as shown in (17) figure 2. Figure 16.b. shows the model.
Adamantane, C10H16
Assembling of the adamantane molecule model can be made several ways, for instance as shown in (13) figure 2, combining two rows of 5 ballonets, three of 4 and three of 2, or as in figure 16. a, using three rows of five and four of two ballonets.
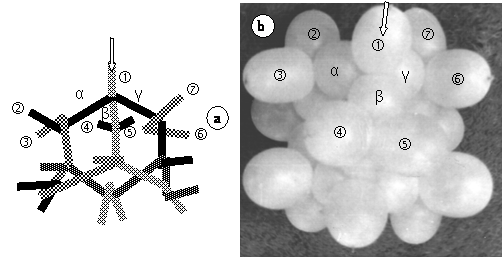
Figure 16. Model of the adamantane molecule. For better understanding, another combination of ballonet rows was chosen in a, than in (13) figure 2. Encircled numbers to ‡ symbolize C-H bonds, while Greek letters, C-C bonds. Arrows allow better comparison of figures a. and b.
Unsaturated hydrocarbons
Ethene (C2H4), also oxygen, formaldehyde, diborane
The model of C2H4 is made of two rows, 3 ballonets each, as seen in (18) figure 2. After crossing one end of each row, the resulting tetrahedral orientation must be forced into a parallel alignment of the middle ballonets, by crossing the other ends of the strings. The two ballonets of the double bonds can be considered two σπ hybrid orbitals. Figure 17.a shows a general view of the model, while 17.b is an almost perpendicular view to the double bond.
In 17.c the diagonals of the C-H bonds (ballonets) are drawn, to demonstrate the slight enlargement of C=C-H angles as well as the shortening of the C-C distances. Figure 17.d shows the Stuart-Briegleb model of truncated spheres of hydrogen (H) and carbon (C) atoms (white circles and black lines) superimposed on the ballonet model (photograph).
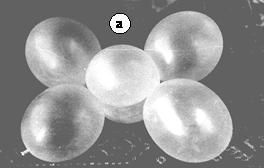
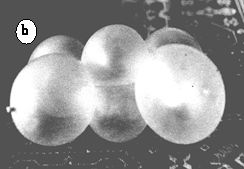
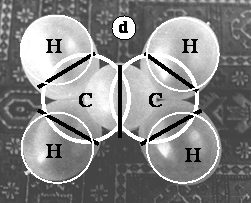
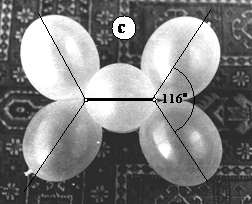
Figure 17. Model of the ethene molecule. a. general view of the model; b. view of the double bond; c. frontal view with bond angles; d. comparison between the Stewart (white circles and black lines) and the ballonet model (photograph)
The ethene model depicts also the oxygen (O2) molecule, the four peripheral ballonets figuring nonbonding orbitals instead of C-H bonds. In the case of H2C=O only the ballonets at one end are modelling nonbonding orbitals, while in diborane each ballonet of the double bond comprises a proton.
Cis- and trans-2-butene (C4H8)
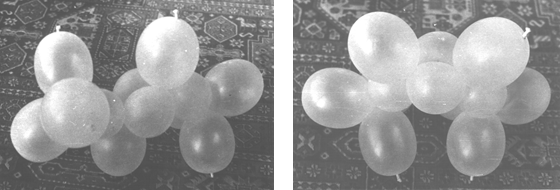
Figure 18. Models of trans and cis-2-butenes.
Two strings of four ballonets along with two pairs are needed to assemble the model, as shown in (20) figure 2. Photographs of trans and cis forms are shown in (21) figure 2. The only constructive difference between the two forms is opposite twisting of the strings at carbon cores 2 or 3.
Cyclobutadiene (C4H4)
The model shown in figure 19 is assembled as (21) figure 2.

Figure 19. Model of cyclobutadiene (isomere of tetrahedrane)
Acetylene (C2H2), also nitrogen, hydrocyanic acid
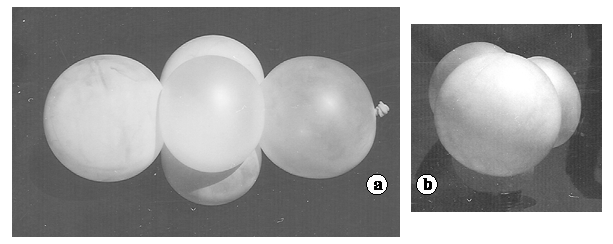
Figure 20. Ballonet model of C2H2, N2 or HCN. a. Perpendicular view to the molecular axis. b. View along the molecular axis
The acethylene model is somewhat difficult to construct, since a knot has to be made on a fiver string as may be seen in (19) figure 2. Some attempt may fail by ballonet burst. Figure 20 shows the model of the acetylene molecule.
The linearity of the molecule is obvious and results almost automatically. The three ballonets of the triple bond can be considered σπ2 hybrids.
Discussion
The most important features of some of the usual models are listed in table I.
Table I. Purposes and achievements of physical molecular models
|
No. |
The model |
Relative bond lengths |
Rotation around σ bonds |
Bond angles |
Space filling |
Tensions in rings and double bonds |
|
1. |
Ball and stick |
fixed |
no |
fixed (as projected) |
no |
no |
|
2. |
Ball and spring |
fixed |
no |
fixed |
no |
yes |
|
3. |
Stick and stick |
fixed |
yes |
fixed |
no |
no |
|
4. |
Skeleton |
fixed |
yes |
fixed |
no |
no |
|
5. |
Dreiding |
fixed |
yes |
fixed |
no |
no |
|
6. |
Stewart-Briegleb |
fixed |
yes |
fixed |
yes |
no |
|
7. |
Bent |
fixed |
no |
result when assembling |
yes |
no |
|
8. |
Elastic spheres |
result when assembling |
yes |
result when assembling |
yes |
can be felt when assembling |
Examining this table it may be seen that the only model which shows all properties: rotation around the single bond, automatic orientation of the valences (bond angles), change of bond angle and length in double bonds, tensions in molecules, is the elastic sphere orbital model, in this case, the ballonet string model.
What are the shortcomings of the ballonet string model?
¸ The most important is the short life of balloons.
¸ Although rotation is possible around the single bond, this rotation is limited, because the seal between ballonets may be affected and air can flow from one ballonet to another.
¸ There is some friction when ballonet strings are put in place, and some adjustments can be necessary in order to achieve the desired shape.
¸ Assembling of ballonet strings with the desired volume and shape needs some exercise.
What are the practical advantages of this model?
¸ All sorts of balloons are easily available as well as pump to inflate them.
¸ Balloons are cheap.
¸ Students can build models at home and play with them studying the structure of molecules.
Conclusions
Physical models of molecules have a specific role in chemical education because they are not only visualizing in three dimensions the form of molecules, but they also can be touched, rotated and examined from all angles. Furthermore, tensions can be felt when assembling the models, in molecules with double bonds or small cycles. Therefore, the use of this model as demonstration and/or classroom or laboratory exercise is recommended in high schools and colleges.
References