Studies into the Factors that Affects the Service Integrity of Galvanizing Kettle
Muhammed Olawale Hakeem AMUDA, Ayisat Adebukunola LAWAL,
Olakunle Wasiu SUBAIR and Ganiyu Ishola LAWAL
Department of Metallurgical and Materials Engineering, University of Lagos, Lagos, Nigeria
Engineering Materials Research Department, Nigerian Building and Road Research Institute, Km. 10 Idiroko-Ota Road, Ota Ogun State Nigeria
E-mail(s):Mohrabiat2000@yahoo.com, bukkielawal@yahoo.com, w6668zubair@yahoo.co.uk gilawal@yahoo.com
Abstract
The finding of studies into the factors that affects optimal performance of galvanizing kettle is presented in this paper. The production schedule and history of a failed galvanizing kettle for 3-consecutively years in a hot-dip galvanizing industry was collated and analyzed. The analysis of the collated data revealed that average galvanizing temperatures for the 3-year under review were 483.9°C (2003), 482.25°C (2004) and 482°C (2005) respectively. The amount of flux, and dross produced in the corresponding years were 169.15kg and 31.6 tons, 56.31kg and 10.5 tons and 101.14 and 18.91 tonnes for 2003, 2004 and 2005 respectively. During these years, zinc consumed averaged 647, 334 and 446 tonnes respectively.
Stochiometry evaluation of flux, and dross in a hot-dip galvanizing process determined flux required as 60 kg/year and dross produced as 90 slabs / month.
The study revealed that the lifespan of galvanizing kettle is greatly reduced by temperature fluctuation, dross formation, excess flux additions and combinations of these factors.
It is recommended that improved service integrity of galvanizing kettle is assured at galvanizing temperature of 460°C, flux consumption of 0.15 – 0.20kg daily and constant removal of dross at 3-5 slabs per day. This reduces the formation of skim lines, which leads to pits on the walls of the kettle.
Keywords
Service integrity; galvanizing temeperature, Flux; Dross; Galvanizing kettle
Introduction
Ferrous materials find application in transportation, telecommunication, health services, civil and building construction, manufacturing and allied industries [1-3]. They are particularly prominent as structural element and roofing material in the built environment because they are relatively cheaper and are flexible in manifesting different structural properties by the modification of their structure through any of the various structural transformation techniques [4]. However, ferrous materials typified by steel have one major draw back. Steel greatly depreciates when exposed to unfriendly environment [5]. The consequences of such depreciation may involve loss of active production time, accident, economic loss and at times may be exceedingly fatal. This is more important in situations where steel is used as load carrying element; bridges, mast and towers, rebar reinforcement, corrugated roofing sheet [6]. Corrosion in these systems is better prevented from setting in than making effort at stopping it once it is initiated. There are two principal methods of preventing corrosion. In one of the methods, the metallic surface is insulated from the corrosive medium by some form of protective coating. Some of these coatings are various types of paints and vanishes, metallic films having good corrosion resistance and artificially thickened oxide films. In the other method, though quite expensive, metal/alloy which has inherent corrosion resistance is employed [7]. The option of using protective coatings is often adopted. The most widely used metallic coatings are electroplating and dipping. For instance, in the built environment, protection of plumbing, transport systems and corrugated roofing sheet made of ferrous material is effected through a process known as hot-dip galvanizing. In the process, iron and steel is coated with a thin zinc layer at a temperature of about 460°C in a molten bath of zinc known as galvanizing kettle. Galvanizing as a means of corrosion control is used majorly in chemical processes, utilities, pulp and paper, automobile and transportation industries [8].
The critical unit in the galvanizing process is the galvanizing kettle (the zinc pot). This unit made of low-carbon low silicon steel is rectangular in framework and is where the actual galvanizing is accomplished. The chemical reaction of the galvanizing process takes place in the kettle. After several cycles of operation, the inside of the kettle is attacked by molten flux, zinc and dross. The attack is aggravated by temperature fluctuations. This leads to erosion and builds up of deposit and ultimately cracks in the kettle [9]. The success of the galvanizing process is highly affected by the integrity of the galvanizing kettle. Therefore, there is motivation to investigate the loss in integrity of the galvanizing kettle as a result of the interaction of these factors with a view to determining optimum conditions of flux, dross and production temperature necessary for enhancing the lifespan of a typical galvanizing kettle.
The present work discussed the findings from studies into the effect of factors such as amounts of flux, dross and production temperature on the service integrity of galvanizing kettle. The object of the studies is to evaluate means of improving the lifespan of the galvanizing kettle. The investigation involved studying the effect of temperature on localized wear of the kettle and that of intermetallic dross on the capacity of the galvanizing kettle. Besides, the contribution of flux to intermetallic dross is reported.
Material and Method
Sourcing of Material
Production data were collected from Galvanizing Industries Limited, Oba Akran Road, Ikeja Industrial Estate, Nigeria. The data collected spanned three year production schedule. Other materials are analar grade saturated solution of sodium carbonate, 6M nitric acid, 0.1M silver nitrate, sodium hydroxide.
The constituent components of the galvanizing process are the zinc pot (galvanizing kettle), the flux box, the zinc box, pyrometer, inner guide and chemical. The critical unit in the galvanizing process is the zinc pot. A cross-sectional representation of the galvanizing kettle is given in Figure 1.
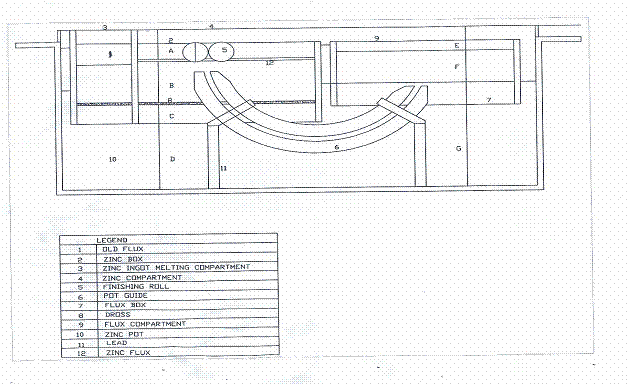
Figure 1. Sectional view of the zinc pot [10]
Table1. Dimensions of important parts
|
A. |
6 ¾ “ |
171mm |
Depth of Coating rolls |
|
B. |
9” |
229mm |
Depth of Zinc layer |
|
C. |
½“ |
13mm |
Depth of Dross layer |
|
D. |
231/8” |
587mm |
Depth of Lead layer |
|
E. |
5 ½ “ |
139mm |
Depth from edge to Coating rolls |
|
F. |
7” |
178mm |
Depth of Flux layer |
|
G. |
267/8” |
683mm |
Depth of Lead layer |
Each component of the galvanizing process is discussed in detail.
The zinc pot is a low carbon, low silicon rimmed steel constructed via electric arc welding using compatible low carbon low silicon electrode. It is rectangular in shape of dimension 2400mm x 1450mm x 950mm and 32mm thick. It is used for holding the various compartments as well as the chemicals for heating during galvanizing operation.
The Flux Box is made of stainless steel or low carbon low silicon steel with firebrick lining inside to protect it from chemical attack. The flux box is used to inject flux during the galvanizing operation.
The Zinc Box is of the same composition as the galvanizing kettle, it is electric arc constructed, rectangular in shape and 32mm thick.
The Pyrometer
A chrome-alumel thermocouple with compensating wire of copper/constantan system was used as a temperature measuring device.
The inner Guide is also made of the same steel as that of the flux box and galvanizing kettle. It has the following specifications-thickness of steel plate-25mm, width of steel plate-80mm, number of steel plate-15 pieces of both the upper and lower guide. It is curved and thus allows the easy passage of the black sheet during the galvanizing process.
Method
Collection of Failed Galvanizing Kettle Data
A dis-used galvanizing kettle was picked from the galvanizing industry. The production data associated with the dis-used galvanizing kettle were collated, tabulated and analyzed (Tables 2-4).
Table 2.Zinc consumption, dross output and galvanizing temperature for 2003
|
MONTH |
ZINC (SLABS) |
DROSS (SLABS) |
TEMPERATURE (°C) |
|
Jan |
3382 |
198 |
480 |
|
Feb |
3427 |
194 |
468 |
|
Mar |
2096 |
97 |
479 |
|
Apr |
3669 |
262 |
477 |
|
May |
902 |
68 |
483 |
|
June |
1907 |
126 |
489 |
|
July |
2980 |
279 |
491 |
|
Aug |
2791 |
139 |
492 |
|
Sept |
2982 |
190 |
491 |
|
Oct |
1780 |
111 |
489 |
|
Nov |
- |
- |
- |
|
Dec |
- |
- |
- |
|
Total |
25916 |
1664 |
4839 |
A zinc slab = 25kg
A dross slab = 19kg
Zinc consumption = 647,900kg
Dross output = 31,616kg
Average temperature=483.9°C
Table 3.Zinc consumption, dross output and galvanizing temperature for 2004
|
MONTH |
ZINC (SLABS) |
DROSS (SLABS) |
TEMPERATURE (°C) |
|
Jan |
2758 |
194 |
472 |
|
Feb |
1574 |
60 |
473 |
|
Mar |
1491 |
53 |
476 |
|
Apr |
961 |
41 |
493 |
|
May |
1790 |
68 |
490 |
|
June |
1696 |
10 |
485 |
|
July |
937 |
63 |
494 |
|
Aug |
2148 |
65 |
475 |
|
Sept |
- |
- |
- |
|
Oct |
- |
- |
- |
|
Nov |
- |
- |
- |
|
Dec |
- |
- |
- |
|
Total |
13355 |
554 |
3858 |
A zinc slab = 25kg
A dross slab = 19kg
Zinc consumption = 333,876kg
Dross output = 10,526kg
Average temperature = 482.25°C
Table 4. Zinc consumption, dross output and galvanizing temperature 2005
|
MONTH |
ZINC (SLABS) |
DROSS (SLABS) |
TEMPERATURE (0C) |
|
Jan |
2688 |
190 |
483 |
|
Feb |
3088 |
186 |
482 |
|
Mar |
2462 |
148 |
490 |
|
Apr |
1392 |
57 |
485 |
|
May |
1706 |
67 |
482 |
|
June |
1066 |
46 |
476 |
|
July |
1175 |
93 |
475 |
|
Aug |
2256 |
111 |
475 |
|
Sept |
- |
- |
- |
|
Oct |
431 |
8 |
490 |
|
Nov |
1500 |
89 |
489 |
|
Dec |
- |
- |
- |
|
Total |
17824 |
995 |
4825 |
A zinc slab = 25kg
A dross slab = 19kg
Zinc consumption = 445,600kg
Dross output = 18,905kg
Average temperature = 482°C
The amount of flux-ammonium chloride-dispersed in a fixed quantity of intermetallic dross was determined through wet analysis. The analysis involve identification of both ammonium and chloride ions to confirm the presence of flux in the dross and then using simple stoichiometry equation to determine amount of flux dispersed in a known quantity of dross.
Identification of Ammonium, Chloride Ions and Calculation of Amount of Flux in Dross
1g of the dross sample was placed in 25ml beaker. 2ml 6M sodium hydroxide solution was added. The mixture was covered with a watch glass stucked with a piece of red litmus paper underneath it. The content was warmed gently. The red colour of the litmus paper changed to blue, thus confirming the presence of ammonium ions.
2g of dross sample was weighed and placed in 25ml beaker. 10ml saturated solution of sodium carbonate was added and warmed until the sample dissolved. 3 drops of the warmed solution was added to 10ml of water and acidified with nitric acid. 2ml of 0.1M silver nitrate solution was added. A white precipitate of silver chloride formed immediately. This confirms the presence of chloride ions.
![]()
1g of dross sample was dissolved in 6M sodium hydroxide in a 100ml beaker. The mixture was agitated and allowed to settle. Ammonia gas was evolved. The volume of hydrochloric acid produced was determined through titration with sodium hydroxide. The titre value of Hcl was 59.9ml. Therefore, the volume of sodium hydroxide consumed by ammonium chloride to liberate ammonia (NH3) gas is (60-59.9) ml = 0.1ml
Results and Analysis of Result
Analysis of Result
The stoichiometry equation for the reaction is
![]()
1 mole NH4CL requires 1 mole NaOH to liberate 1 mole NH3(g).
Applying Avogadros law, this implies that
1 volume ![]() requires 1 volume NaOH
requires 1 volume NaOH
Thus 0.1ml ![]() consumed 0.1M NaOH
consumed 0.1M NaOH
But 1 mole ![]() = 53.5g
= 53.5g
Recall that 1 slab of dross = 19kg (19000g)
If 1g of dross ![]() 0.000535 kg of
0.000535 kg of ![]()
then 19000g of dross ![]() 0.10165kg of
0.10165kg of ![]()
For year 2003, the average dross output was 31,616kg, therefore, contribution of flux to dross is given as
![]()
=169.15 kg of ![]() (flux)
(flux)
At 25kg/bag of![]() ; this implies that about 6 bags of
; this implies that about 6 bags of ![]() is lost to
dross in the production year 2003.
is lost to
dross in the production year 2003.
For year 2004, the dross output was 10,526kg, therefore contribution of flux to dross
10,526kg (dross)![]()
=56.31kg of ![]()
At 25kg/bag of ![]() in the production year 2004; about 2
bags of
in the production year 2004; about 2
bags of ![]() was
lost to dross formation.
was
lost to dross formation.
This low volume of flux in dross was apparently because there was no production in 2004 for four months (September - December) (Table 2).
For 2005:
Dross output=18,905kg
Contribution of flux to dross
18,905 kg (dross)![]()
![]()
= 101.14kg of ![]()
This translates to about 4 bags of flux at 25kg/bag.
Estimation of the Wear Rate of the Galvanizing Kettle
The wear rate of the galvanizing kettle was estimated in terms of the corrosion rate.
The corrosion rate was evaluated using the formular proposed by Krisher[11].
Corrosion Rate (C.R) = ![]() (1)
(1)
where W = weight loss; ![]() = Material density in g/cm3;
T = Exposure time in hours; A=surface area of exposure ; K is a constant
and can be varied to calculate corrosion rate in various units:
= Material density in g/cm3;
T = Exposure time in hours; A=surface area of exposure ; K is a constant
and can be varied to calculate corrosion rate in various units:
K = 3.45 x 106 Corrosion rate in mils/year, A (cm2)
K = 8.75 x 104 Corrosion rate in millimeter/year A (cm2)
K = 5.35 x 105 Corrosion rate in mil/year A (1n2)
Dimension of new kettle = 240cm x 145cm x 95cm
Thickness = 3.2cm
Density of mild steel =7.76g/cm3
Effective volume of new kettle = outside volume – inside volume (2)
Outside Volume = 240 x 145 x 95
= 3.31 x 106 cm3
Inside Volume = (240 - (3.2 + 3.2) x (145 – (3.2 + 3.2)) x (95 – (3.2 + 3.2))
= (240 – 6.4) x (145 – 6.4) x (95 – 6.4)
= (233.6) x (138.6) x (88.6)
= 2.87 x 106 cm3
Effective volume = (3.31 x 106 – 2.86 x 106) cm3
=4.37 x 105 cm3
Dimension of old kettle = 240 x 145 x 95
Thickness = 2.5cm
Effective volume of old kettle =outside volume – inside volume
Outside Volume of disused kettle = (240 x 145 x 95) cm3
= 3.31 x 106 cm3
Inside volume of disused kettle
= (240–(2.5 + 5.2)) x (145–(2.5 + 2.5)) x (95–(2.5 + 2.5))
= (235) + (140) x (90)
= 2.96 x 106 cm3
Effective volume of old kettle = 3.45 x 105 cm3
Weight of new kettle = ![]() (3)
(3)
where V1 = effective volume of new kettle; ![]() = is as
defined in equation (1)
= is as
defined in equation (1)
![]() weight of
new kettle = 7.76 x 4.37 x 105
weight of
new kettle = 7.76 x 4.37 x 105 ![]() = 33.91 x 105 =
3.39 x 106g
= 33.91 x 105 =
3.39 x 106g
Similarly,
Weight of old kettle = ![]()
where V2 = effective volume of old kettle
![]() weight of old kettle = 7.76 x 3.45 x 105
weight of old kettle = 7.76 x 3.45 x 105
![]() =
26.77 x 105g = 2.68 x 106g
=
26.77 x 105g = 2.68 x 106g
Weight loss = weight differential between new and old kettle
= 7.76 (V1 – V2)
= 3.39 x 106 – 2.68 x 106
= 7.14 x 105 kg
Surface area of kettle in cm2
A = 2 (LH + BH + LB)
= 2 [(240 x 95) + (145 x 95) + (240 x 145)]
= 2 (22,800 + 13,775 + 34,800)
= 2 (71,375)
= 142,750 cm2
Corrosion rate in millimeter per year as defined by Krisher [10] equation (mm/y)
= ![]()
Taking the 1st year (2003) as reference
For the 1st year C. R. = ![]()
Assuming a production hour of 8 hours per day and production days of 22 days per month.
Therefore time T in hours = 8 x 22 x 12 = 2,112 hours
For the 1st year (2003)
C. R.=![]()
C. R.=![]()
C. R.=![]() =
= ![]()
C. R.=26.81 mm/y
For the 2nd year=![]()
![]() =13.40 mm/y
=13.40 mm/y
The corrosion for the 2nd year=corrosion rate 1st year+estimated 2nd year rate
=16.81+13.40 = 40.12 mm/y
For the 3rd year (2005)
C. R.=![]()
C. R.=8.94 mm/y
The actual corrosion rate for the 3rd year = 40.21+8.94
=49.15 mm/y
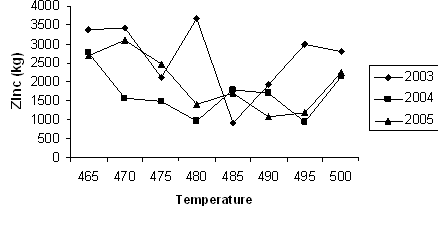
Fig 2. Zinc consumption against galvanizing temeprature
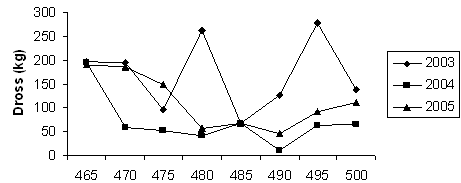
Fig 3. Dross output against galvanizing temperature
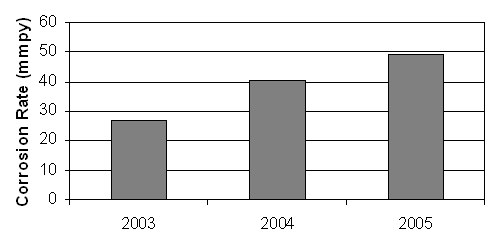
Fig.4. Corrosion rate of galvanizing kettle over a 3 year cycle
Discussion
Figure 2 shows the zinc consumption against galvanizing temperature for the production years 2003, 2004 and 2005 respectively. The figure revealed that the production year 2003 consumed the highest quantity of zinc (3600 slab) at galvanizing temperature of 478°C. Subsequently, the consumption of zinc decreases as the galvanizing temperature increases. It is apparent from the figure that between 478°C and 480°C, there is a sudden drop in the amount of zinc consumed. Further reduction in zinc consumption as galvanizing temperature increases was gradual. The same flow pattern was observed in the production year 2004. However, in the production year 2005, there was a sudden increase in the consumption of zinc at galvanizing temperature of about 484°C. Afterward, there was reduction in the amount of zinc consumed.
The general observation in the three year production periods was that the amount of zinc used for galvanizing decreases as hot-dip galvanization is taken to higher temperature. This seems to be desirable in contrast to the standard recommended galvanizing temperature of 460°C – 470°C since at the recommended temperature, the formation of intermetallic dross is intense and this may create a situation where more zinc is consumed. This apparently explain why more zinc is consumed at lower galvanizing temperature.
However, galvanizing at too high a temperature increase the energy cost. The saving generated in zinc consumption at high temperature might be offset by the high energy cost incurred at too high a temperature. Besides, galvanizing at too high a temperature leads to increase in the formation of intermetallic dross with very high intrusion of flux. This increases the quantity of flux applied during hot-dip galvanizing process.
Figure 3 is the curve of dross produced against changes in galvanizing temperature for the three years under study. The curve revealed that the highest volume of intermetallic dross was produced in the 2003 production year while the least was produced in 2004. Juxtaposing Figures 2 and 3 revealed that the higher the amount of zinc consumed the higher the amount of dross produced irrespective of the galvanizing temperature. The amount of zinc consumed has a greater influence on dross produced than the galvanizing temperature.
The distribution of flux in the intermetallic dross varies across the three years under study. In 2003, 169.15kg of NH4Cl flux was dispersed in about 31.6 tonnes of dross as zinc ammonium chloride (ZAC) crystals. This is equivalent to 6 bags of NH4Cl at 25kg/bag. The amount of flux dispersed in intermetallic dross in 2004 was equivalent of 2 bags and in 2005, equivalent of 4 bags of NH4Cl flux was dispersed in the dross. The very low quantity of flux dispersed in dross in 2004 was due to the very low production schedule experienced in that year.
Dross is a heterogeneous product skimmed from the surface of liquid metals. They are not formed as single liquid phase but rather are mixtures of precipitated solid and liquid compounds with substantial proportions of mechanically trapped mother metal from the underlying metal bath [10].
When the steel reaches galvanizing bath temperature, the zinc reacts with the steel to form series of zinc-iron alloys. Some of these zinc iron alloy crystals floats off the surface of the steel and enters the zinc bath. Over time, these zinc iron crystals coalesces and sink to the bottom of the zinc bath. This forms a mushy layer called dross.
As the dross is heavier than zinc and lighter than lead, it floats in between the zinc and lead in the kettle. This forms skim line on the walls of the kettle and gradually erodes the wall and the kettle corrodes in the process. It is also possible that, the dross may get caught in or on the workpiece and form a rough lumpy deposit; or it may stick to the surface of the galvanizing as it is being withdrawn from the bath. The dross may equally give a galvanized coating a gritty appearance (dross pimples).
The wear rate of the galvanizing kettle was estimated in terms of the corrosion rate. Figure 4 is the chart of the rate at which galvanizing kettle deteriorated with the production year. The kettle had a corrosion rate of 26.81mmpy in 2003, 40.12mmpy in 2004 and progressively increased to 49.18mmpy in 2005. The trend displayed by the rate of corrosion was as a result of deposits that have accumulated over three years that reduced the effective thickness of the kettle from 32mm to less than 15mm. The practical implication of these rates is that after the first year, the galvanizing kettle was expected to have thinned by 2.6cm and that by the third year, the kettle would have thinned out. In actual fact, this was what happened, as by 2005, the galvanizing company had replaced the galvanizing kettle with a new one.
The lower the galvanizing temperature the better as long as dipping can be guaranteed [11]. Irregular bath temperature / temperature fluctuations as a result of poor burner system causes creep and fatigue which affects the integrity of the kettle over a period of time. The amount of strain developed in the material becomes a function of temperature / time and a system subject to creep may be deemed to have failed when its dimensions have changed by some predetermined value.
In the present study, it is apparent that galvanizing temperatures in the three years under review had not been uniform. This situation induces expansion and contraction stresses in the galvanizing kettle and it is apparently responsible for the cracks observed in the kettle. Thus, temperature fluctuations over a range of values increases the susceptibility of galvanizing kettle to cracks. It is therefore, a good practice to avoid temperature fluctuations in galvanizing process.
Conclusions
The effects of factors such as dross, temperature and flux on the service integrity of galvanizing kettle have been investigated. The study revealed that the lifespan of the galvanizing kettle is reduced by temperature fluctuation, dross formation, excess flux additions and combination of these factors. The average galvanizing temperature in the three years under review range between 482-4830C. Galvanizing at this range of temperature lead to excessive consumption of flux and high rate of dross formation. This leads to the formation of skim lines which creates pits on the walls of the galvanizing kettle affecting the service integrity of the kettle in the process.
The study revealed that the service integrity of galvanizing kettle is improved at galvanizing temperature of 4600C, and this has the tendency to reduce volatilization of the zinc which helps in reducing zinc consumption in galvanizing process, removal of 3 slabs of dross daily and maintaining the contribution of flux at about 0.15kg/day (about 2 bags of flux per year).
Acknowledgements
The authors are grateful to the management of Galvanizing Industries Limited, Oba Akran Road, Ikeja Industrial Estate, Lagos, Nigeria for making available the record of their production schedule and history which facilitated the early completion of the study.
References
1. Brandes, E. A. and Brook, G. B. (Editors), Smithells Metals Reference Book, 7th Edition, Butterworth – Heinemann Ltd. Oxford. Pp. 15-30, 1992.
2. Lewis, G., Selection of Engineering Materials, Prentice Hall Inc. Englewood Cliffs. NJ. 1990.
3. Dieter, G. E., Mechanical Metallurgy 3rd Edition, McGraw-Hill Book Co. New York. 1986.
4. Brooks, C. R., Principles of Heat Treatment of Plain Carbon and Low Alloy Steels ASM International Materials Park, OH. 1995.
5. Callister, W. D. Jr., Materials Science and Engineering – An Introduction, 4th Edition, John-Wiley & Sons Inc. New York, pp. 860, 1997.
6. American Galvanizers Association, Hot-Dip Galvanizing for Corrosion Prevention www.galvanizeit.org. 2006.
7. Cohen, M., Societal Issues in Materials Science and Technology, Materials Research Society, Bulletin, pp. 3-12, 1994.
8. ACI Committee 222, Corrosion of Metals in Concrete, American Concrete Institute, 1985.
9. Yodogawa Steel Corporation, Galvanizing (Hot-Dip) Pg. 4-12. www.yoooko.co.jp/galvalue, 2006.
10. Lawal, A. A., Investigation into the Factors that Affect the Integrity of Galvanizing Kettle, Unpublished B.Sc. (Hons) Final Year Project Research. Department of Metallurgical and Materials Engineering, University of Lagos, Lagos, Nigeria, Pp 60, 2006.
11. Krisher, A. S., Technical Information Regarding Corrosion Testing www.alspi.com/cpntst.htm, 2006.