Production of Ethanol Fuel from Organic and Food Wastes
Uduak George AKPAN*, Adamu Ali ALHAKIM, and Udeme Joshua Josiah IJAH
Department of Chemical Engineering, Federal University of Technology, P.M.B. 65, Minna, Niger State, Nigeria.
Department of Microbiology, Federal University of Technology, P.M.B. 65, Minna, Niger State, Nigeria.
E-mail(s): ugaekon@yahoo.co.uk, udemijah@yahoo.com
(* Corresponding author)
Abstract
Production of ethanol fuel from organic and food waste has been carried out with the singular aim of converting the waste to useful material. To achieve this, the conversion of organic waste (Old newspapers) and food waste (maize) were respectively carried out via acid and microbial hydrolysis, which yielded 42% and 63% fermentable sugar wort. This was then converted into ethanol by fermentation process using Sacchromyces ceverisiae. 95% ethanol was obtained by fractional distillation of the fermentable wort and the total volume of ethanol produced from 2,500 grams of the organic and food wastes was 0.86 liters.Fermentation Kinetic parameters were evaluated. Considering the percentage fermentable sugar yield from the biomasses in study, it is more economical to produce ethanol from food waste (maize) than old organic waste (old newspaper).
Keywords
Ethanol fuel; Organic waste; Biomass; Fermentation; Conversion; Distillation.
Introduction
Studies [1-4] have revealed that there are attempts to develop alternative source of energy so as to conserve the dwindling reserves of crude oil and fossil fuels. The world population and increased urbanization have directly or indirectly influenced the energy demand. Presently, in most countries of the world energy is derived from crude oil and fossil fuels. However, the problems associated with petroleum and fossil fuel sources are that they are limited in supply and cannot be renewed, hence deplete on usage. It is therefore evident that there is the need to search for alternative fuel sources which can be renewed with time; and ethanol has been found as one of such fuel sources.
Ethanol produced by fermentation has been found to serve considerably as transportation fuel for cars, trucks and trains [5]. The production of ethanol is not limited, but constantly replenished by growing plants and is advantageous over petroleum as a source of fuel in that petroleum source is steadily depleted with usage. Ethanol fuel has not been fully exploited because gasoline has been available, cheap and easy to produce. However, there is an increasing demand for fuel today and the price differential between ethanol and gasoline is getting narrower [6].
There are lots of other alternative fuels such as methanol, methane, natural gas, propane, hydrogen, etc. Nevertheless, the remarkable characteristics of ethanol distinguish it as the best alternative fuel for automobile. It has high latent heat of vaporization, high octane number and rating, and emission of toxic compounds on its combustion is low [6]. Though ethanol has a lower heating value of about 60% of typical regular gasoline, this low heating value is compensated for, by high latent heat of vaporization of 361Btu/Ib (839.686kJ/kg) which is more than twice that of gasoline, which is about 140Btu/Ib (325.64kJ/kg). Thus, when ethanol and gasoline are respectively burned in correct stoichiometric ratios, they have about equal volumetric efficiency. When gasoline is burned, it produces water, carbon dioxide, carbon monoxide and other impurities such as oxides of sulphur and nitrogen, and heavy metals. On the other hand, pure ethanol is burned to produce carbon dioxide, water, and a much lower amount of carbon monoxide. Hence, ethanol will be a better replacement for gasoline.
Ethanol is used as an automobile fuel by itself and can be mixed with gasoline to form gasohol. Ethanol can be burned in the millions of existing vehicles engines with little or no modifications. According to Rural Industries Research and Development Corporation (RIRDC) [7], ethanol may be used as a fuel in several ways:
a) At level up to 10% ethanol may be blended with gasoline (E-10), and used in most modern cars engines with no modifications;
b) In Brazil a blend of approximately 22% ethanol (E22) in gasoline is used in many vehicles with only minor modifications of engines;
c) In both USA and Brazil, some vehicles use pure, hydrous ethanol (an azeotrope of 96% ethanol and 46% water). In each case vehicles require engines and fuel systems that are designed with the high levels of ethanol in mind. Ethanol is also used in fuel cell for stationary power and automobiles.
Ethanol can be produced by fermentation of any organic materials rich in carbohydrate. Akande and Mudi [2] studied the kinetic model for ethanol production from cassava starch by Saccharomyces cerevisiae yeast strain. The results obtained indicated that the model could predict optimum fermentation performance using sugar in cassava starch as the substrate and an indicator for selection or evaluation of potential yeast strains. In this present study, wastes (maize-food remains and old newspapers) were used in ethanol production via two routes; microbial and acid hydrolyses.
Several factors affect the production rate of ethanol by fermentation, and a suitable mathematical description of the fermentation process has been developed. This helps in interpreting fermentation measurements with a view to early detection of poor fermentation performance, the ability to predict future fermentation behaviour and application to design and advanced control of fermentation and optimization [8].
For cell concentration, X, the logistic model was derived as follows;
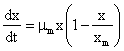 (1)
(1)
Where, µm is the maximum specific growth rate with respect to the fermentation conditions as the form of the Monod relationship with the following boundary conditions:
t = 0, X = Xo, S = So, P = 0
By integration of equation 1, the kinetic model can be formulated. The biomass production rate yields the following equation [9]:
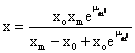 (2)
(2)
Equation (2) shows the relationship between biomass and fermentation time, which is used to fit experimental data of biomass concentration.
A delay of ethanol production was found compared with the cell growth, and little ethanol was produced during the yeast lag phase. Therefore, a parameter of the lag time, ∆t, was introduced to describe the delay of ethanol production to cell growth, and the equation of ethanol production rate was modified as equation (3)
![]() (3)
(3)
Equation (3) can be integrated using two estimated parameters from equation (2) µm and Xm, and the Model is described by equation (4).
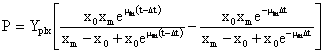 (4)
(4)
The equation describing the substrate consumption rate takes into account two aspects, the sugar consumption in the formation of biomass and the maintenance of biomass. Wang et al [9] described the consumption rate of sugar as:
![]() (5)
(5)
Combining equations (1), (3) and estimated parameters, equation (5) can be integrated and the sugar consumption equation can be represented by equation (6):
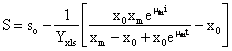
![]() (6)
(6)
Table 1: Definition of terms and symbols use in equations
|
Term/Symbol |
Definition (Units) |
|
X Xm X0 Ms ∆t T P S S0 Yp/x Yx/s µ µm |
Biomass concentration (g/L) Maximum biomass concentration (g/L) Initial biomass concentration (g/L) Maintenance coefficient (g sugar/g biomass h) Lag time (h) Time (h) Produced ethanol concentration (g/L) Fermentable sugar concentration (g/L) Initial fermentable sugar concentration (g/L) Yield coefficient of ethanol on biomass (g ethanol/g biomass) Yield coefficient of biomass on sugar(g biomass/g sugar) Specific growth rate (h-1) Maximum Specific growth rate (h-1) |
These parameters are necessary for the interpretation of fermentation measurements and prediction of future fermentation behaviour. Therefore, the present study was aimed at converting wastes (maize-food remains and old newspapers) to a valuable product, ethanol fuel via two routes; microbial and acid hydrolyses. The study considered the enormous amount of food and organic wastes thrown away at the refuge dump, and the eventual environmental pollution caused by it, and therefore embarked upon the conversion of these wastes into a useful product, ethanol. By doing this, the environment will be cleaned
Material and Method
Collection and processing of samples
Old newspapers collected from the wastes bin of Bank of the North, Minna, food wastes - maize paste collected from Nana’s Restaurant, Bosso, Minna, Nigeria were the major raw materials used for the experimental work. The materials were collected in black polythene bag and transported to the Laboratory where the experiments were performed.
The cellulose substrate (old newspapers) was cut into small pieces using a pair of scissors, while the starch substrate (the food left over of plain cooked maize) was sun dried and crushed into small particles using mortar and pestle.
Conversion process
Four liter of water was added to 1200g of the starch substrate. 90g of dried grounded barley malt was added to the starch substrate. The mixture was cooked at 50oC for 60 minutes. Thereafter, the temperature was raised to 75oC, where termanyl (an enzyme) was added and the temperature of the mash was maintained at 75oC for 40minutes to gelatinize the mash. The temperature of the mash was then raised to 90oC for an hour; this was to aid the liquefaction of the mash. The mash was then cooled to 60oC where 300g of barley malt, and fungamyl were added and the temperature maintained at 60oC for 50minutes. The malt slurry was constantly stirred throughout the process, and then cooled to room temperature. The slurry was filtered to remove solids and to recover the sugar solution. 1300g of cellulose substrate (old newspapers) was mixed with 1650g of diluted sulfuric acid, and was heated to 100oC for 6h. The hydrolyzed material was soaked in water and drained, several times; the solid residue was then dewatered, and soaked in 40% sulfuric acid for 4 hours. The material was then dewatered, dried and re-mixed with 40% sulfuric acid and maintained at 100oC for 3h. The content was filtered to remove solid and recover the sugar/acid solution. The sugar/acid solution was neutralized using sodium hydroxide.
Preparation of yeast culture
Two hundred grams (200g) each of sugar and flour were respectively added to 250ml of water. The solution was heated to 100oC for 45minutes, and then cooled to room temperature. 20g of dried yeast (Saccharomyces cerevisiae) was added to the mixture. The yeast culture was maintained in the refrigerator at 20oC until required.
Fermentation and distillation
The two worts (cellulose and starch) were filtered, mixed and diluted with water to adjust the initial sugar concentration. Sulfuric acid was also added to adjust the pH to 5.0. Seven airtight fermentation set-ups were made. The activated yeast culture was added and the entire wort was equally distributed into the seven air tight fermentation set up. The entire set up was kept at room temperature 28±2oC until when the wort ceased from bubbling and the yeast cake had sunk to the bottom. The fermented wort was immediately distilled, thereby adding benzene to obtain 95% ethanol.
Determination of sugar, ethanol and biomass concentrations
The concentration of sugar in the wort was determined at a 12-hour interval using sugar automatic analyzer (Model YP-2378 P1). The thin plastic sample tubing from the glucose analyzer was inserted into the wort sample tube. The sugar analyzer was switched on, the sample key was pushed. The sugar concentration was displayed within a minute, and the value was recorded.
Every 12hours, sample of the fermented wort was taken for ethanol concentration analysis. The fermented wort was distilled and the specific gravity of the fermented wort was determined using hydrometer.
The biomass concentration was determined using spectrophotometer (Model 63 SPEC-nm) at a 12hours interval. A small quantity of the sample was taken and placed in the spectrophotometer. The absorbance of the sample was taken at a wavelength of 630nm using water as a blank.
Results
The results on the production of ethanol from organic and food wastes are shown in Table2:
Table 2: Conversion of organic and food wastes to fermentable sugar
|
Substrate |
weight of substrate (g) |
specific gravity |
%Sugar after Conversion |
|
Old newspaper Maize |
1300 1200 |
1.889 1.277 |
42 63 |
The volume of ethanol produced from 2500g of the substrate (old newspaper+maize) was 0.86L.
The results of fermentation kinetics studies of ethanol production from
organic and food waste using Sacchromyces cerevisiae yeast are shown in Tables 3 and 4.
Table 3. Biomass, ethanol and sugar concentrations produced from organic and food wastes at different time intervals
|
Time (h) |
Concentration (g/L) |
||
|
Biomass |
Ethanol |
Sugar |
|
|
0 12 23 36 46 60 70 82 96 108 118 132 144 150 |
0.7 1.5 3.2 4.0 6.0 6.6 7.0 7.5 7.7 8.0 8.3 8.3 8.1 8.1 |
0.0 1.0 5.6 15.3 24.4 36.4 40.5 42.4 43.2 43.5 44.2 44.0 44.0 44.0 |
82 75 72 65 58 45 35 26 19 11 8 5 4 2 |
Table 4: Kinetic parameters estimated from experimental data on organic and food waste fermentation.
|
Kinetic Parameter |
Sugar (Organic/Food Waste) |
|
µm (h-1) Xm (g/L) Yp/x (g/g) Yx/s (g/g) ∆t (h) Ms (h-1) |
0.0922 8.0275 5.5775 0.2200 15.47 0.1144 |
Discussion of Results
Conversion process
The quantity of sugar obtained after conversion of old new papers and maize substrates were 42% and 63% respectively. These values are slightly different from those reported in the literature. For instance Mathewson [6] reported 46% and 66% fermentable sugars respectively from papers and food substrates. The slight departure from literature for the old newspapers might be as a result of reaction residence time, concentration of acid used, pre-hydrolysis of the substrate and size reduction. In the case of maize substrate, the variation might be traced to the ratio of the malt to substrate used for conversion. Since the process is microbial, the actual ratio of malt to substrate can be attained after several attempts.
Fermentation kinetics
The fermentation profiles of Sacchromyces cerevisiae on organic and food waste are shown in Table 3. Based on the experimental data, some kinetic parameters including yeast maximum specific growth rate (µm), maximum biomass concentration (Xm), the yield coefficient of biomass on sugar (Yx/s), the yield coefficient of ethanol on biomass (Yp/x), lag time (∆t) and maintenance coefficient (Ms), were estimated by mathematical software (polymath 5.0) using equations 2, 4, 6 respectively. The estimated values of the kinetic parameters are given in Table 4.
The estimated values of Yx/s, and Ms are the same as reported by Wang [9], those of µm and Xm are respectively +2.4% and +1.1% of the values of Wang [9], while those of Yp/x and ∆t are respectively -1.2% and -1.1% of Wang [9]. The variation of the estimated Values of µm, Xm, Yp/x and ∆t of this experiment with that obtained by Wang may be due to fermentation condition such as temperature, pH, initial sugar concentration, inoculation, nature and type of fermenter, and the nature of the substrate used.
Ethanol yield from organic and food wastes
The volume of ethanol produced from 2500g of organic and food waste was 0.86 litres. As reported in literature by Mathewson (1980), that a ton of 40% - 60% fermentable sugar substrate can produce 70 -100 gallons of ethanol. This means that substrate of 2500g, and containing 40% - 60% fermentable sugar can produce a maximum ethanol yield of about 0.93 litres and a minimum yield of 0.65 litres. The experimental yield of 0.86 litres of ethanol from 2500g of organic and food waste is well within acceptable range. The experimental results obtained in this work showed that both the conversion and fermentation processes were optimal.
Conclusions
The conversion process employed for old newspapers (which are basically made up of cellulose) was achieved by acid hydrolysis, where the complex cellulose structure was broken down into simple fermentable sugar. The amount of fermentable sugar that was obtained from the acid hydrolysis of the old newspaper was obtained to be 42%, with only slight variation from the standard.
The maize substrate (basically made up of starch), was converted into simple fermentable sugar by microbial process. The microbial hydrolysis of maize substrate yielded 63% with a very minute variation from the standard.
The production of ethanol fuel from organic and food waste yielded 0.86 liters of 95%ethanol from 2500g of old newspapers and maize substrates, which were respectively converted to 42% and 63% fermentable sugar. Kinetic parameters and predicted data were estimated from experimental data using a non-linear kinetic model. Apart from the fact of relieving the environment of pollution which may be attributed to old newspaper, to have economic production of ethanol fuel, it is best to use food waste (maize) which gives a higher percentage of fermentable sugar.
References
1. Adeniyi, O. D.; Kovo, A. S.; Abdulkareem, A. S. and Chukwudozie, CEthanol Production from Cassava as A Substitute for Gasoline. J. Dispersion Sci. Technol. 28:501-504. (2007).
2. Akande, F. H. and Mudi, K. Y., Kinetic Model for Ethanol Production from Cassava Starch by Saccharomyces cerevisiae Yeast Strain. Proceedings of the 35th Annual Conference of NSChE, Kaduna, Nigeria. (2005)
3. Akpan, U. G. Acid Demethylation of Agricultural Waste (Citrus Peel). A. M. S. E 46(6): 33-42. (2003)
4. Akpan, U. G.; Kovo, A. S.; Abdullahi, M. and Ijah, U. J. J.Production of Ethanol from Maize Cobs and Groundnut Shell. AU J. T. 9(2): 106-110. (2005).
5. Morrison R.T and Boyd R.T., “Organic Chemistry” 6th Edition, 213-235, 1143-1195, Prentice Hall of India Private limited. (1992)
6. Mathewson, S. W., “The manual for home and farm production of alcohol fuel,” pp1- ppss, j.a. diaz publications. (1980)
7. Rural Industries Research and Development Corporation, “Wood for alcohol fuels status of technology and cost / benefit analysis of farm forestry for bioenexgy”, RIRDC Publication No 02 / 141.(2002)
8. Boulton, R., “The prediction of fermentation behaviour by a kinetic model, PP40-45, Am. J. Anol vitic. (1996)
9. Wang, D., Xu Y.Hu. J. and Zhaog, Fermentation kinetics of difference sugars by apple wine yeast(no.4).Sacharomyce cere vistae, J. Institute of Brewing, 110, 340-346. (2004)