Synthesis and Characterization of Fatty Acid Methyl Ester by In-Situ Transesterification in Capparis Deciduas Seed
Raghunath D POKHARKAR, Prasad E FUNDE, Shripad S JOSHI Shirish S PINGALE
Jain irrigation systems Ltd. Jalgaon India
Ravikiran and Tribal Woman Development Institution Maharashtra India
Raghunath D POKHARKARRAVIKIRAN, 31 Gharkul, Opp. to Sangamner College, Sangamner, Ahmednagar 422 605
Email(s): joshi.shripad@jains.com, gharkul31@rediffmail.com shirish.pingale@rediffmail.com pef@rediffmail.com, pdfardi@rediffmail.com
Abstract
(FAME) Fatty acid methyl ester is made virgin or used vegetable oils (both edible and non-edible) and animal fats. Fatty acid methyl ester operates in compression ignition engines like petro-diesel. Fatty acid methyl ester can be blended in any ratio with petroleum diesel fuels. It can be stored just like the petroleum diesel fuel. Petrodiesel can be replaced by biodiesel due to its superiority. It has various advantages. The seeds of Capparis deciduas are found to contain non-edible oil in the range of about 63.75 %. The percentage of biodiesel yield increases with concentration of KOH as a catalyst. The aim of this article is to demonstrate the cost effective new source of energy by single step reaction i.e. production of oil by combining extraction and reaction of extract with the mixture of alcohols. In this article the effect of catalyst concentration, time, water content and temperature on in-situ transesterification is studied to obtain optimum yield and Fatty acid methyl ester (Biodiesel) Fuel characterization tests show the striking similarity of various physical & chemical properties and campers to ASTM standards.
Keywords
Biodiesel; KOH; in-situ transesterification; Catalyst; Capparis deciduas.
Introduction
Bio-diesel is an environment friendly fuel,[1,2]which has almost no sulphur, no aromatics and has about 10% built-in oxygen. It is fatty acid ethyl or methyl ester. Advantage of Fatty acid methyl ester Apart from energy [3]security, employment generation, economic gain, social security, bio-fuels have enormous environmental benefits being superior in many aspects than conventional source of energy [4]i.e. petrol and diesel.
Biodiesel, a most important need of future can be obtained by in-situ transesterification reaction. The triglycerides are easily converted to monoalkyl esters of long chain fatty acids by this technique.
The reaction of reactive extraction is carried out by following three stages[5]
(a) Conversion of triglycerides to diglycerides
(b) Conversion of diglycerides to monoglycerides
(c) Conversion of monoglycerides to biodiesel ester
Capparis deciduas tree Capparis deciduas seed


Figure 1. Figure 2.
In in-situ transesterification seed powder is extracted with alcohol where alcohol acts as a solvent as well as reactant. This process reduces the cost of final product as this process has less number of unit operations. It is the best non-renewable source of energy with good environmental impact and easy recovery.
The aim of this article is to demonstrate the cost effective new source of energy by single step reaction effect of reaction parameters on in-situ transesterification is studied to obtain optimum yield and Fatty acid methyl ester (Biodiesel) Fuel characterization tests show the striking similarity of various physical & chemical properties and campers to ASTM standards.
Material and Method
The seeds of Capparis deciduas collected, cleaned and dried. The seeds are then grinded to fine powder by using heavy duty electric mixer of high rpm. Ten grams of seed powder was used as a starting material. It was mixed with mixture of Methanol and Ethanol. The in-situ transesterification with continuous stirring was carried out by adjusting 300 rpm oscillations. The heat is given by hot plate by keeping at 800 C for about 45 minutes. The solid cake and mother liquor were separated by vacuum filtration. A rotary evaporator was used for separation of solvent. The oil fraction separates at 800C. The oil content was preserved in airtight containers and used for further analysis.
The moisture content of dry seed powder and oil extracted by reactive extraction was obtained by Karl Fischer Titrator, µaquacal100, manufactured by Analab Scientific Instruments Pvt. Ltd. During in-situ transesterification various concentrations of potassium hydroxide were used as a catalyst along with the mixture of alcohols. The reaction time was finalized for optimum yield is 45 minutes. The reaction was carried out at different temperatures. The temperature 800C gives maximum yield where as the oil was also separated at 800C by rotary evaporator. The water quantity also affects the rate of reaction considerably. Increase of aqueous medium reduces the yield of reactive extraction. The observed yield is maximum without addition of water. The separation of various components was studied by Thin layer Chromatography. The best solvent for separation was found to be Acetic Acid, Pet. Ether and Ethanol with volume ratio 0.75: 7.25: 2.00. The silica gel suspended in chloroform and a pinch of plaster of pairs was used for preparation of chromo plate. The spots were observed on chromatogram by keeping dry developed plate in iodine chamber. The areas of spots were calculated by usual method. These observations were used for calculation of percentage yield.
Results and Discussion
The percentage of biodiesel from Capparis deciduas seeds was found to be 63.75 % percent (in oil fraction). The moisture content of oil obtained by Karl Fischer method was 0.56 percent and the moisture content of seed powder is around 0.4 percent. The optimum temperature for in-situ transesterification is 80°C. The agitation was achieved by keeping 300 rpm oscillations continuously for 60 minutes optimized time.
The reactive extraction was studied for different concentrations of KOH, temperature and time intervals so as to obtain optimum conditions.
TLC study of oil fraction obtained without heating and constant stirring did not show
The chromatographic plate shows the positions of spots. All plates shows different number of spots. Some plates show only two spots.
The figure of effect of concentration of KOH clearly shows that the yield of biodiesel changes with change in the concentration of catalyst. The optimum yield of biodiesel was found at 0.08 N Concentration of KOH.
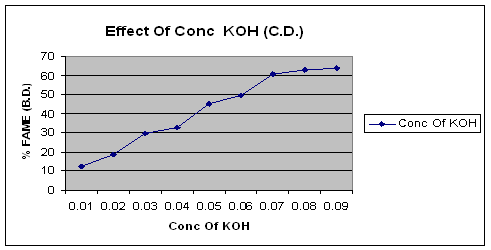
Figure 1. Effect of concentration of KOH
The figure of effect of water quantity on reactive extraction clearly shows that the yield of biodiesel changes with change in the concentration of water. The optimum yield of biodiesel was found without water.
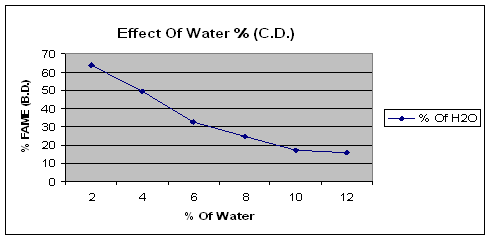
Figure 2. Effect of water
To date, most of the research has focused on the transesterification at near boiling point of alcohol used. A few works reported the reaction at room temperature. The synthesis of ethyl ester of Capparis deciduas oil in batch reactor of 63.75% conversion optimum molar ratio of 1:2, 1:6 for Capparis deciduas oil at 75C to 80oC and 25 bar was studied. At 40oC, the relatively low conversion to alkyl esters evident due to the subcritical state of alcohols. Between 40oC, the onversion reaches 20%. These results show good agreement with previous studies however, at 80oC, a conversion of of 63.75 %fatty acid methyl ester (Biodiesel) was observed. In this work, the effects of reaction temperature on the production yield and methyl ester concentration are presented in figure 3. The temperature had effect on methyl ester concentration. As results above, 80oC temperature is considered to be the optimum temperature.
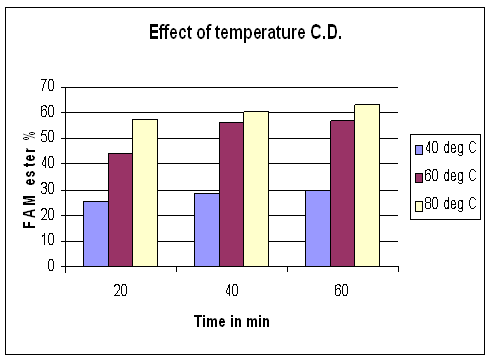
Figure 3. Effect of reaction temperature
The fatty acid methyl ester (Biodiesel) of the Capparis deciduas are tested for Acid numbers, Sulphated ash %, Sulfur, wt %, max, Phosphorus, wt%, Free/ Total glycerin, Cloud point ASTM D2500, Kinematic Viscosity, cSt@40c, Copper strip corrosion rating, max, corrosion, Cetane Number (see Table 1). These results are tabulated and compared with ASTM standard the conclusions are drawn on the basis of standard. These plants can be used for the preparation of Fatty acid methyl ester (Biodiesel) which will be suitable for use replacement of diesel without any change in engine.
Table 1. Fatty acid methyl ester (Biodiesel) Fuel characterization tests show the striking similarity of various physical & chemical properties and campers to ASTM standards.
|
No |
Performance Characteristics |
Std.(FAME) B.D.ASTM |
Capparis deciduas |
|
1 |
Acid numbers, KOH/gm |
0.80 |
0.430 |
|
2 |
Sulphated ash % |
0.05 |
0.023 |
|
3 |
Sulfur, wt %,max |
0.05 |
0.005 |
|
4 |
Phosphorus, wt%, |
0.001 |
0.002 |
|
5 |
Free/ Total glycerin. |
0.020 |
Nil |
|
6 |
Cloud point ASTM D2500 |
< 210 |
+10 |
|
7 |
Kinematic Viscosity,cSt@40c mm2/S |
1.9-6.0 mm2/S |
1.01 |
|
8 |
Copper strip corrosion rating,max |
No.3 max. |
<3 |
Conclusions
(FAME) Fatty acid methyl can be produced from the seeds of Capparis deciduas by in-situ transesterification. The optimum concentration of KOH is 0.08 N. The biodiesel fraction from oil content was found 63.75 % at 800C and 300 rpm oscillations for 60 minutes time and normal atmospheric pressure without addition of water in the reaction mixture. The harmful organic reagent like n-hexane was not at all used in this method. Hence this technique is environment friendly. The Biodiesel obtained has low cost and low viscosity. One can support the replacement of petrodiesel by biodiesel; as it is easily recovered. The catalyst used is KOH which is cheap and easily available. Fatty acid methyl ester (Biodiesel) Fuel characterization results are tabulated and compared with ASTM standard the conclusions are drawn on the basis of standard. These plants can be used for the preparation of Fatty acid methyl ester (Biodiesel) which will be suitable for use replacement of diesel without any change in engine.
Acknowledgements
Authors are thankful to “Ravikiran Rural and Tribal Woman Development Institution” for sample collection and financial support.
References
1. Foidl N, Eder P (1997) Agro-industrial exploitation of J. curcas. In. Biofuels and Industrial Products from Jatropha curcas, Gübitz GM, Mittelbach M, Trabi M (Eds), Dbv-Verlag, Graz, Austria
2. Foidl N, Foidl G, Sanchez M, Mittelbach M, Hackel S (1996) Jatropha curcas L. as a source for the production of biofuel in Nicaragua; Bioresource Technol. 58, 77-82
3. Report of the committee on development of Bio-fuel as formatted by the Planning Commission of India
4. Vivek and A.K Gupta (2004), “Biodiesel Production from Karanja Oil”, Journal of Scientific & Industrial Research Vol. 63, 39-47.
5. Encicar, J.M., Gonzalez, J.F., Rodriguez, J.J. and Tejedor, A. (2001) Biodiesel Fuels from Vegetable oil; Transesterification of Cynara cardunculus L. Oils with Ethanol, Energy & Fuels.