Thermal and Ablative Properties of Ipns and Composites of High Ortho Resole Resin and Difurfurylidene Acetone
Tariq S. Najim, Amel M. Naji and Mahmood M. Barbooti
Chemistry Department, College of Science, Mustansirya University, Baghdad, Iraq.
And Department of Applied Chemistry, School of Applied Sciences, University of Technology, Baghdad, Iraq.
E-mail: brbt2m@gmail.com
Abstract
High ortho resole resin was prepared by condensation of phenol with excess of formaldehyde in the presence of magnesium oxide as catalyst. Reaction of furfuraldehyde with acetone in basic medium led to difurfurylidene acetone (DFA). Their interpenetrating polymer network (IPNS) were obtained by the reaction of predetermined quantities of difurfurylidene acetone and high ortho resole using p-toluene sulphonic acid (PTSA) as curing agent. The thermal behavior of the resins was studied using thermogravimetry (TG) under ambient and nitrogen atmospheres over a temperature range of (25-1000 Cº). It was observed that the IPN of 20% DFA – 80% resole has higher thermal stability than that of resole alone and the decomposition temperature was higher by 80 Cº. This behavior was attributed to highly cross linked structure and thermally stable backbone of ploy difurfurylidene acetone due to formation of ladder structure.
Impregnation of chopped fiber glass type (E) with the polymeric solutions was used to prepare their composites, and the ablative properties were investigated according to ASTM E-285 –80. It was observed that the IPN of (DFA- resol) perform better than the resole composite alone.
Keywords
IPN, Thermal and ablative properties, Resole, Difurfurylidene acetone.
Introduction
Phenolic resin is a widely used industrial material because of it's good heat resistance, electrical insulation, dimensional stability, and chemical resistance. Improvement of its properties, especially heat resistance, is required particularly for the industrial field. Many approaches have been tried to improve the heat resistance of phenolic resin, either by means of after–cure [1] or an increase of hardening agent content [2]. On the other hand, furan mon omers are often used for preparing thermo-resistant polymers [3]. It was found that these resins have many applications as floor and silo coverings, anti corrosion coatings, wood-particle adhesives, organic concretes and concrete binder and also used in graphitization [4].
Difurfurylidene acetone can be crosslinked using acidic catalyst leading to insoluble and infusible polymer network [5]. Therefore, it is possible to make interpenetrating polymer networks (IPNs), with other polymers. On the other hand, better mechanical,thermal and flame retardance properties have been obtained from novel phenolic resin-silica nanocoposites [6-8].
In this study, new IPNs based on difurfurylidene acetone and high ortho resol resin were prepared in the presence of p-toluene sulfonic acid and their thermal stability were investigated by TG.
A polymeric composites of high o-resole resin only and an IPN sample besed on 20% DFA and high ortho resole were prepared by using fiber glass type -E as a reinforcement, the polymeric composite were molded by heat and pressure using hot hydraulic press, then their ablative properties were examined using flame test.
Materials
Furfural (Purified by vacuum distillation) [ 9] (Baker ), formaldehyde solution (37%), p-toluene sulphonic acid (Riedel–De Haen), phenol, calcium stearate, magnesium oxide, polyvinyl butyral, γ-aminopropyl–triethoxy silane, benzene, acetone, toluene, petroleum ether (Merck), absolute ethanol, chopped fiber glass type E-glass, length (3-5) cm (Fluka).
Preparation of High-Ortho Resol Resin (I)
High ortho resol was prepared according to a procedure reported earlier [10] with some modifications. A four-necks round bottomed flask equipped with mechanical stirrer, temperature controller and condenser was charged with 94 gm (1 mole) of phenol, 4.8 gm (0.12 moles) of the catalyst magnesium oxide and 40 gm of toluene. The solution was stirred and heated to about 100oC, after which, 98 gm (1.2 moles) of aqueous formaldehyde 37% were added to the reaction mixture over three hours duration. After all the aqueous formaldehyde solution had been added, the mixture was azeotropically distilled for about 1.5 hrs under atmospheric pressure to a temperature of about 110oC. The heat source was then removed and the distillation continued under reduced pressure for about half an hour. The solid content of final products was evaluated. A sample from the bulk was dried using shallow small dish in a vacuum oven for 24 hrs at 30oC, a sample of the resin was heated for 5 hrs at 180oC. The product was characterized by IR and studied by TG analysis.
Preparation of Difurfurylidene Acetone (DFA) (II) [11]
(0.5 mole, 48 mg) of redistilled furfural was mixed with (0.25 mole, 14.5 gm) acetone. The reactants were mixed well and cooled in an ice bath to 5oC, then poured on a cold (100) ml of NaOH solution (20%), and then the reaction was carried on at room temperature for 1 hour with continuous mixing until a yellowish precipitate was formed. The product was filtered washed with water to get rid of the traces of NaOH, recrystallized from petroleum ether, then dried in a vacuum oven for 3 hours at 25oC, and was characterized by I.R.
Preparation of Ipns Based On High-Ortho Resol Resin and Difurfurylidene Acetone (III-VII)
The new IPNs were prepared by reacting the predetermined quantities of difurfurylidene aceton (II) and high ortho resol resin (I) with p-toluene sulphonic acid (P.T.S.A.) as hardner as shown in Table 1. The difurfurylidene acetone, high ortho resol and (P.T.S.A.) were mixed gently for about 10 min. at room temperature. Samples from the bulk were cured at 80oC for 2 hrs and at 180oC for 5 hrs for the thermoanalytical studies. An uncured sample (V) was used in the preparation of composite material for ablative test, Table 3. While all the cured samples (III – VII) were analysed by TG, Figs. 1-5 and Table 2.
Table 1: Typical Formulae Used In The Preparation Of The Ipns Based On Dfa And High Ortho Resol Resin
|
Sample Code |
Resol (gm) |
DFA (gm) |
PTSA (gm) |
|
III |
- |
1 |
0.10 |
|
IV |
2 |
0.4 |
0.24 |
|
V |
2 |
0.6 |
0.26 |
|
VI |
2 |
0.8 |
0.28 |
|
VII |
2 |
1 |
0.30 |
Preparation of the Polymeric Phenolic Composites
Composite samples were prepared by using chopped fiber glass type-E as reinforcing fiber impregnated with a polymeric matrix based on the following resins:
1. Phenol – formaldehyde high ortho resol resin;
2. High ortho resole resin and difurfurylidene acetone, (V).
The chopped fiber-glass type E was treated [12] with 2.0% by weight of γ-aminopropyltriethoxysilane as coupling agent at pH-5 aqueous solution and dried in air for one day. Subsequently, the fibers were dried under vacuum at 50oC for an additional ten hrs. The polymeric matrix was prepared by mixing the resins under study with the 12% by weight of polyvinylbutyral (P.V.B) [13], and 2% by weight of calcium stearate (Ca-St) as a release agent, by using mechanical stirrer for about 15 min. at room temperature. Then the viscosity of the polymeric matrix was measured by using (German Standard) (DIN-4) cup. The viscosity can be adjusted by adding absolute ethanol in order to achieve a viscosity range of 45-50 s which is suitable for the impregnation of the fiber glass. The silane treated chopped fiber-glass was then impregnated in the polymeric matrix. The fiber glass should form about 60% of the composite weight. The prepared wet polymeric composite samples were spread by hand into small portions and then dried in an oven for 4-5 hrs at 50oC. The humidity content should not exceed 4-5%. The polymeric composites were molded by using hot hydraulic press.
Thermal Analysis
The thermogravimetric (TG) analyses of the IPN’s samples (III-VII) based on resol-difurfurylidene acetone were carried out using Stanton - Redcroft (TG 760) series (1983), on 4-8 mg samples of the cured resins. The samples were heated at a rate of 20oC/min under ambient atmosphere. The TG curves are shown in Figs. 1-5. The TG measurement of the prepared high ortho resol (I) resin was carried out on Thermo Haak, Exstar 6000, TG-DTA 6300, S11 (Seiko Instruments) using 2-5 mg samples of the cured resins and exposed under programmed heating rate of 20oC/min from (25-800oC) under inert atmosphere (N2 gas). The thermogram is shown in Fig. 6. The numerical evaluation data are listed in Table 2.
Ablative Testing
The ablative tests were carried out according to ASTM E 285-80, by this test, composite burn through time, erosion rate, char rate and insulation indices were determined. The set up consists of a welding torch, equipped with a mixture of oxygen – acetylene gases (1:20 by volume). The insulation index was calculated by dividing the time by original thickness of the specimen in mm. The char rate was also determined as reciprocal of the insulating index (ablative index x 1000). The erosion rate was calculated by dividing the original thickness of the specimen by the time to burn through. The prepared composite samples were tested and the ablative parameters obtained are listed in Table 3.
The FTIR spectra were recorded for the samples using a Shimadzu 8300 FTIR spectrophotometer.
Results and Discussion
High ortho resol resin which is suitable for the fabrication of glass fiber reinforced composites was prepared by reacting (1)mole of phenol with 1.2 mole of an aqueous solution of formaldehyde under azeotropic distillation conditions in the presence of divalent electropositive metal catalyst and an intermediate PH 4-7. The high ortho reactive site are attributed [14] to formaldehyde reaction with phenolic hydroxyl and methylol groups. The presence of hemiformals has been supported by NMR [15]. Magnesium oxide was used as a catalyst in this study which forms a chelating metal complex with the phenolic hydroxyl group. The IR spectrum of the resol resin (I) showed abroad high intensity band at 3300 cm-1 which is attributed to the phenolic OH and the OH of methylol groups and band at 3066 cm-1 due to aromatic C-H stretching. It also showed C-H aliphatic stretching asymmetrical and symmetrical bands at 2933, 2889 cm-1respectively, and bands at 1620, 1610 and 1450 cm-1 due to C=C aromatic stretching vibrations. The spectrum showed two bands, the first at 1087 cm-1which is related to dibenzyl ether and the second band at 1049 cm-1due to C-O stretching vibration of the hydroxymethyl group.
The IR spectrum of difurfurylidene acetone [16] showed the characteristic bands at 1600, 1555, 1485 and 1390 cm-1. The first band at 1600 cm-1was attributed to the ethylenic linkage and the other three bands are characteristics of an α-olefinic carbon substituted furan. The band at 3140 cm-1 is attributed to C-H stretching of the furan ring and the bands at 2915 and 2830 cm-1 are attributed to C-H stretching vibrations. Absorption bands at 1020 cm-1 ascribed to the C-O-C, at 1625 cm-1 to the C=O groups.
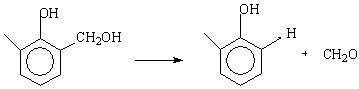
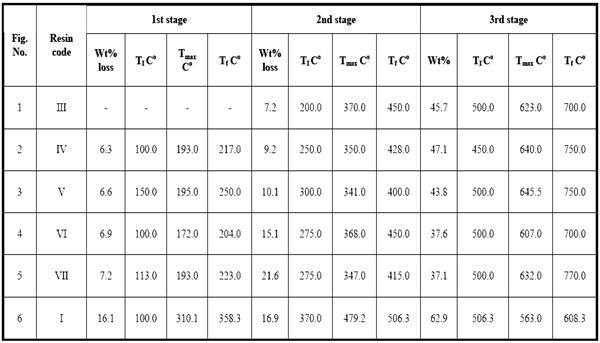
Table 2 shows the data obtained from the thermal decomposition stages of IPNs samples (III-VII). The corresponding thermographs are shown in Figs. 1-5. The TG studies of IPNs samples (III-VII) showed that they behave at the beginning of heating like resol alone. These IPNs lose about 14% of their weight at (130-240) oC, and reach the maximum rate of decomposition at 193 oC. The resol alone typically showed the same weight loss percentage as in Fig. 6. This can be attributed to the loss of formaldehyde as suggested by Maciel et al. [17]. The thermal decomposition of cured resol has been investigated by solid NMR spectroscopy where the methylol groups are expelled [14].
After the loss of formaldehyde, the resulting polymers (IPNs) become relatively stable and behave thermally some what like cured DFA alone (III) (Fig. 1), which has a maximum rate of decomposition at 623 oC. The first decomposition stage of cured DFA occurred at a maximum rate at 370oC. It was observed that this temperature undergoes a sort of lowering so that this decomposition in IPNs sample of DFA – resol, occurs at Tmax(350)oC in the presence of 15% DFA (Fig. 2), Tmax341oC in the presence of 20% DFA (Fig. 3), Tmax368oC in the presence of 25% DFA (Fig. 4) and Tmax(347)oC in the presence of 30% DFA (Fig. 5). Therefore, the IPNs of resol and DFA present desirable results, after the loss of formaldehyde, where the resulting IPNs have good thermal stability and regular behavior. The maximum rate of decomposition of resol alone was found to be at 563 oC in idle atmosphere (N2), (Fig. 6). Meanwhile, the increment of DFA in the IPNs of (resol and DFA) improved the thermal stability and made it decompose at a higher temperature of 645oC in the presence of 20% DFA, sample V (Fig. 5), although it was carried out at oxidizing atmosphere (air). From these results, it is clearly seen that the IPNs of resol-DFA has a higher thermal stability and the decomposition temperature is higher than that of resol alone by about 80oC. This is attributed to the ladder polymer structure which is formed through the crosslinking of DFA in the presence of PTSA. The DFA has two reactive furan rings, when used alone they are suitable for preparing a highly dense polymer. Polymerization in the presence of PTSA results in the formation of solid, non-melting and insoluble furan polymer, dark brown to black in color. This indicates that the cross linking proceeds via the furan double bonds or via the olefinic double bond [3] leading to the formation of ladder polymer structure as shown below in Schemes 1 and 2.
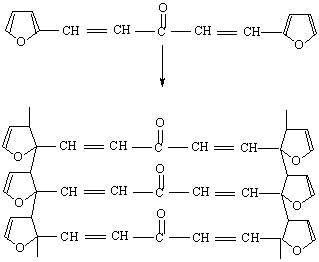
Scheme (1). Crosslinking via the furan ring
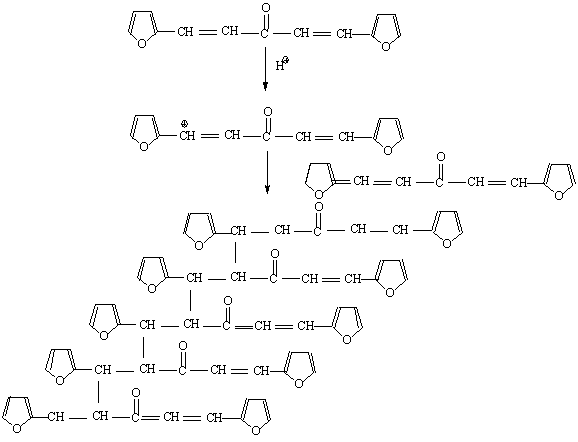
Scheme (2) Crosslinking via the olefinic double bond
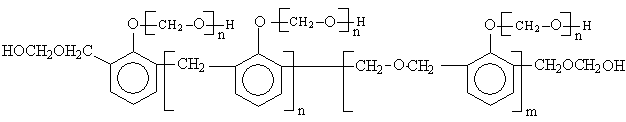 (Resole)
(Resole)
 Table
2: The Thermal Characteristics Of Ipns (III-VII) Cured Dfa and Cured Resole
Table
2: The Thermal Characteristics Of Ipns (III-VII) Cured Dfa and Cured Resole

The ablative characteristics of the polymeric composites investigated and the resulting burn through time, erosion rate, charring rate and insulation indices were listed in Table 3. In general the flame ablation test indicated that the erosion rate and insulation indices exhibited by the samples under study did not vary strongly.
The IPNs of resol – difurfurylidene acetone resin (V) showed interesting results and perform better than the resol composite sample (I). This is due to the fact that furan resins demonstrate specialty properties including high carbon yield and stability at elevated temperature [18]. Further, the pyrolysis of furan resins above 450 Co gives high yield of glassy porous carbon [4].
The good ablative characteristics of the resins under study may also be due to the fact that reinforcing fibers may act as a heat dissipater or provide a much better network for transferring heat in a random manner. This may divert the heat path away from the targeted direction much more than the uncomposited resin would do. Hence the fiber network will actually act as an additional heat barrier. At the same time it will act as a mechanical stabilizer to the charred layers, which protect this porous layer. Thus, will hinder the erosion process and in turn enhance the insulation process by maintaining the thickness of these layers.
Table (3) Insulating ablative parameters for the composites (60/40)% fiber glass to matrix of resol resin and IPNs.
|
Resin code |
Thicken (mm) |
Burn through time (sec) |
Insulation index (sec/mm) |
Erosion rate (mm/sec) |
Char rate (mils/sec) |
|
I |
7.3 |
35 |
4.79 |
0.209 |
8.35 |
|
V |
7.2 |
36 |
5.0 |
0.200 |
8.0 |
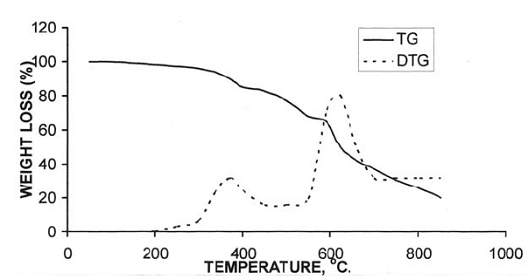
Fig. (1: TG and DTG thermogram of resin(III)
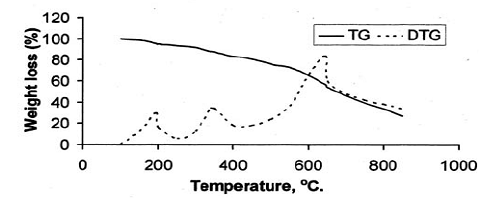
Fig. (2): TG and DTG thermogram of resin (IV)
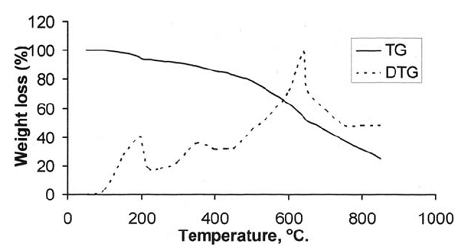
Fig. (3: TG and DTG thermogram of resin (V)
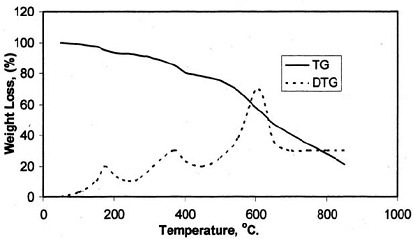
Fig. (4): TG and DTG thermogram of resin (VI)
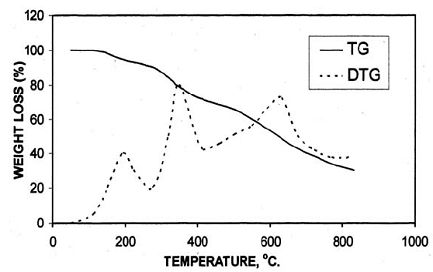
Fig. (5): TG and DTG thermogram of resin (VII)
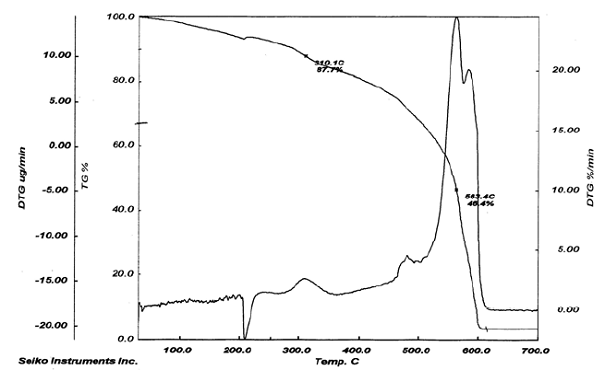
Fig. (6): TG and DTG thermogram of resin(I)
References
1. A. Fukuda, K. Hasegawa and H. Hoiuchi, Kobunshi Ronbunshu, 40, 329,1983.
2. T. Ohta, N. Ando and Y. Goto, Jpn. Kokai Tokkyo Koho, JP 88086746, April 18, 1988.
3. G. Borisov, I. Kraicheva and S. Varbanova, Eur. Polym. J., 19, 827-829 ,1983.
4. K. J. Siegfried, “Furan Polymers”, In Encyclopedia of Polymer Science and Technology, Vol. 7, (H.F. Mark, Ed.), Interscience, New York, PP.432-445, 1967.
5. P. S. Patel and S. R. Patel, Eur. Polym. J., 23, 733-735,1987.
6. Chin-Lung Chiang and Chen-Chi M.Ma, Polymer Degradation and Stability 83,207-214 (2004).
7. Min Ho Choi ,In Jae Chang, J. Applied Polymer Science,Vol.90, 2316-2321,2003.
8. Yu Jian-Ying,Wei Lian-qi and Gao Xian-Kun, J. of Wuhan University Technology Vol.18 No.4, 2003.
9. B.S. Furnass, A. T. Hannaford, V. Rogers, P.W.T. Smith and A. R. Tatchell, “Vogel,s Textbook of Practical Organic Chemistry”, 4th. Ed., Longmans, London, 1986, p. 792.
10. G. L. Brode and S. W. Chow, U.S. Pat. 4,578,448, Mar. 25, 1986.
11. I.V. Kamenski and N. V. Ungurean, Plast. Massy, 8, 17, 1960.
12. C. H. Chiang and J. L. Koenig, J. Polymer Sci.; Polymer Phys. Ed. 20, 2135-2143, 1982.
13. Ninomiya, Hiroaki, Tomioka, Nobuyuki, Terashita, Takeshi, Nagata, Hideo, Kishi and Hajime, U.S. Pat. 6,391,959, May 21, 2002.
14. A. Knop and L. A. Pilato, “Phenolic Resins; Chemistry, Applications and Performance; Future Directions”, Springer – Verlag – Berlin and New York, 1985.
15. P. Knop and E. R. Wagner, J. Polymer Sci., Polymer Chem. Ed., 11, 939, 1973.
16. R.M. Silverstein, G. C. Bassler and T. C. Morrill, “Spectrometric Identification of Organic compounds”, 4th Ed., John Wiley and Sons, New York,1981.
17. Gary E. Maciel, S. Chuang and L. Gollob, Macromolecules, 17, 1081-1087, 1984.
18. W.J Mckillip and E. Sherman, “Furan Derivatives”, In M. Grayson, Ed., Kirk - Othmer Encyclopedia of Chemical Technology, Vol. 11, John Wiley and Sons, Inc., New York , PP. 499-527, 1981.