The Effect of Tertiary Butyl Hydroquinone on the Biodegradability of Palm Olein
Emmanuel ALUYOR1*, Innocent OBOH2, and Charity OKIEIMEN3
1,3 Department of Chemical Engineering, University of Benin, Benin City, Nigeria.
2 Department of Chemical and Petroleum Engineering, University of Uyo, Uyo, Nigeria.
E-mail: *eoaluyor@yahoo.com; obohio2009@yahoo.com
(* Corresponding author)
Abstract
Poor oxidative stability is demonstrated by most vegetable oils especially in industrial situations. Antioxidants are widely used for overcoming poor oxidative stability in vegetable oils. The adverse effect of additives on the overall biodegradability of vegetable oil based industrial fluids could however be a concern. Biodegradability provides an indication of the persistence of any particular substance in the environment. The superior biodegradation of vegetable oils in comparison with mineral based oils has been demonstrated severally, leaving scientists with the lone challenge of finding economic and safe means to improve their working efficiency in terms of their poor oxidative stability. This study investigated the extent to which the use of the antioxidant Tertiary butyl hydroquinone (TBHQ) in palm olein impaired biodegradability, and described the relationship between antioxidant loading and biodegradability. Increased antioxidant loading resulted in a matching decrease in biodegradability. Using the total cumulative oxygen depletion value of pure refined palm olein at the end of the 28 day period as a standard of comparison, a 0.02% concentration of TBHQ in palm olein resulted in a 25% loss in biodegradability; a 2% concentration of TBHQ resulted in a 56.5% loss in biodegradability. At 6% TBHQ concentration, no biodegradation was observed in the palm olein sample studied.
Keywords
Biodegradation; Vegetable oils; Oxidative stability; Antioxidants; Cumulative Oxygen Depletion.
Introduction
The chemical composition of fats and oils and their specific properties have allowed them to find use as foods, fuels and lubricants. Their sources are numerous, encompassing vegetable, animal, and marine sources. All fats and oils have certain characteristics in common. Fats and oils are naturally occurring substances which consist predominantly of mixtures of fatty acid esters of the trihydroxy alcohol or glycerol [1]. Vegetable oils are obtained from oil-containing seeds, fruits, or nuts by different pressing methods, solvent extraction or a combination of these [2]. Crude oils obtained are subjected to refining processes [2, 3]. Examples of fatty acids which combine with glycerol to form fats and oils include oleic acid, palmitic acid, stearic acid, linoleic acid, linolenic acid, erucic acid, lauric acid, and ricinoleic acid, amongst others. The predominance of either a single one or a group of these acids in the ester structure of fats or oils is used in classifying oils into different groups, which expectedly have different properties. These properties provide the possibility of applying vegetable oils for purposes other than edible ones. FOR instance, vegetable oils and fats have been applied as lubricants for a great many years. This is attributable to the fact that due to their chemical composition and bulk properties, they possess superior lubricity and emulsifying characteristics, as well as a low viscosity-temperature variation. Additionally, vegetable oils are by nature non-volatile, and possess good insulating characteristics. Combine this with the favourable electrical properties possessed by some oils it can find application as a fair transformer oil. Other industrial applications similarly exist.
In a comparison of palm oil and mineral based lubricants, palm oil based lubricants were found to be more effective in reducing the hydrocarbon and carbon monoxide emission levels, among other things [4].
The modern day focus on resource and environmental conservation has brought about renewed interest in the use of these “natural oils” for non edible industrial purposes. Their established superiority in terms of biodegradability when compared with mineral oils, as well as the fact that they are renewable and generally non-toxic has focused attention on technologies and methods that would enhance their usefulness as bio fuels and industrial lubricants [5]. Challenges exist however, and the greatest of these remains the low oxidative stability that is characteristic of oils of natural origin. If untreated, oils from vegetable origin oxidize during use and polymerize to a plastic like consistency [5]. Even when they are not subjected to the intense conditions of industrial applications, fats and oils are liable to rancidity [6,7].
The oxidative stability of oil is a measure of the length of time taken for oxidative deterioration to commence. On a general level, “the rates of reactions in auto-oxidation schemes are dependent on the hydrocarbon structure, heteroatom concentration, heteroatom speciation, oxygen concentration, and temperature "[8]. Based on studies carried out, the oxidative stability of refined vegetable oils is found to be determined considerably by the fatty acid composition, the tocopherols content and the carbonyl value [9].
Combating the issue of oxidative instability in vegetable oils for industrial use is a continuing research area. In the United States, for instance, three avenues are being pursued and one of these is the use of various additives and property enhancers [10].
Antioxidants function either by inhibiting the formation of free alkyl radicals in the initiation step or by interrupting the propagation of the free radical chain [11]. In truncating the propagation step, the antioxidants function as hydrogen donors.
Generally, the most popular antioxidants are hydroxyphenol compounds with various ring substitutions. Examples include the common antioxidants, Tertiary Butyl Hydroquinone (TBHQ), Butylated Hydroxy Toluene (BHT), Butylated Hydroxy Anisole (BHA), and Propyl Gallate (PG). They are characterized by possessing low activation energies for the hydrogen donation process. The antioxidant radical which results is stabilized with its local electrons delocalized; hence antioxidant free radicals do not readily initiate other free radicals. They rather even react with lipid free radicals to form stable and complex compounds [11]. Work has been done where the common phenolic anti-oxidants were tested for their effectiveness in improving the oxidative stability of biodiesel obtained from soybean oil [12]. By definition, biodegradation is the chemical transformation of a substance caused by organisms or their enzymes. There are two major types of biodegradation – Primary Biodegradation, which refers to the modification of a substance by microorganisms such that a change is caused in some specific measurable property of the substance [13].
When the term primary biodegradation is used it refers to minimal transformation that alters the physical characteristics of a compound while leaving the molecule largely intact. Intermediary metabolites produced may however be more toxic than the original substrate [14]. Thus mineralization is the true aim. When this happens it is referred to as Ultimate or Complete Biodegradation; which is the degradation achieved when a substance is totally utilized by microorganisms resulting in the production of carbon dioxide, methane, water, mineral salts, and new microbial cellular constituents [13, 15].
Vegetable oils and synthetic esters have a much better biodegradation capacity than mineral oil under aerobic as well as anaerobic conditions [16]. Tests carried out severally indicate that vegetable oils undergo about 70 -100% biodegradation in a period of 28 days [14]. In a specific comparative study carried out by Mecurio et al., Vegetable-Derived Lubricants were established to be in fact more biodegradable than comparable Mineral-Derived Lubricants in the presence of tropical mangrove or coral reef microbial communities [17].
In mineral oils for instance, the toxicity and environmental hazard of a spill is not determined only by the mineral oil base, but also by the numerous additives utilized in the lubricant formulation [16]. Hence, the actual toxicity of even bio lubricants is to a large extent determined by the nature of used additives [16]. In the compilation by Bloch P., it is definitively stated that additives such as antioxidants and pour point depressants tend to reduce the biodegradability and increase the toxicity of the product to the environment and ultimately humans [18].
The aim of this study is to determine the effect of the common phenolic antioxidant, Tertiary butyl hydroquinone on the biodegradation of palm olein.
Material and Method
Palm olein weighing 50g was put into 4 conical flasks each. Each flask containing oil was heated to 70°C and agitated vigorously. While agitation was going on, TBHQ the antioxidant under study was not added to the first conical flask, 0.01g (0.02%), 1.02g (2%) and 3.2g (6%) of TBHQ was added to the second, third and fourth conical flask respectively. The antioxidant was added slowly and allowed to dissolve properly. The four conical flasks were vigorously agitated for an additional 20 minutes to ensure complete dissolution.
Mineral medium preparation
Two solutions are required for the sustenance of the microbes contained in the inoculum obtained from the Ikpoba River of Benin City, Edo State Nigeria.
Solution A was prepared by dissolving 8.50g of Monopotassium dihydrogen orthophosphate (KH2PO4), 21.75g of Dipotassium monohydrogen orthophosphate (K2HPO4), 33.40g of Disodium monohydrogen orthophosphate dihydrate (Na2HPO4.2H2O) and 0.50g of Ammonium Chloride (NH4Cl) in distilled water and the resulting solution made up to 1 litre. Solution B was prepared by dissolving 0.25g of Iron (III) Chloride Hexahydrate (FeCl3.6H2O) in distilled water and made up to 1 litre. 1ml of solution A was mixed with solution B to constitute the mineral medium.
Experimental procedure
The prepared mineral medium was allowed to stand in the incubator at 20°C in the dark for 20 hours. Subsequently, the mineral medium was strongly aerated for about 20 minutes. 5mg of test substance and 5ml of inoculum was added to 1.5 liters of prepared mineral medium in the following manner:
· The measuring cylinder was filled to about 1/3 of the final volume (500ml) with mineral medium. To this were added 5mg of the test substance, and 5ml of inoculum.
· The solution is made up to 1.5 liters in the measuring cylinder using aerated mineral medium, and transferred to a volumetric flask for agitation using the magnetic stirrer.
After preparing the solution containing mineral medium, inoculum and test substance for a given antioxidant concentration, 50ml was withdrawn to determine the initial dissolved oxygen, and the rest dispensed into four BOD bottles. The filled BOD bottles were corked, and placed in the incubator. The Dissolved Oxygen was determined after 7, 14, 21 and 28 days periods. Dissolved oxygen was determined using the Winkler’s titrometric method.
Results and Discussions
Figure 1 shows the graphical plots of cumulative oxygen depletion values for the various samples, thus representing the biodegradation trends with and without antioxidant.
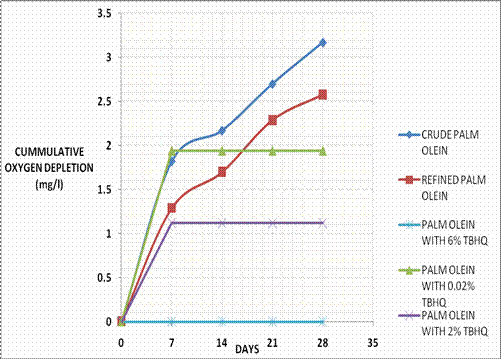
Figure 1. Cumulative Oxygen Depletion with Time for Palm Olein
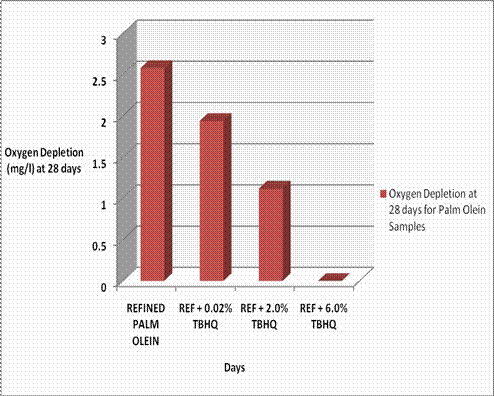
Figure 2. Oxygen Depletion at 28 days for Palm Olein Samples
A summary of the overall obtained result is shown in Figure 2 where the bar charts show the total oxygen consumption for the various samples of palm olein at the end of the 28 day period. Higher oxygen depletion/consumption values at the end of the test period are indicative of higher biodegradability.
In the palm olein sample under study, oxygen consumption was observed to be higher in the samples which did not contain any TBHQ than it was for those containing some amount of antioxidant.
Both crude and pure refined palm olein sample showed high biodegradation rates indicated by their high oxygen usage over the 28 day period. The well confirmed high biodegradation rates of vegetable oils were used as a standard of comparison in this test for vegetable oil samples which contained antioxidant. It may be observed that in this case (palm olein), the oils in their unrefined states showed higher biodegradation rates than in their refined states. The difference in oxygen usage could be attributed to the fact that the oils in their crude states possessed a higher insitu microbial density than their refined counterparts. The test procedure which employs a relatively low microbial density was sensitive to changes in microbial density. Steps in the refining procedure brought about a reduction in the microbial density of the oils. Hence the slightly lower values of oxygen consumptions observed. However both crude and refined oil samples in this case showed high rates of biodegradation.
For all samples, weekly oxygen depletion values were highest in the first week, since the biodegradation process was fastest at the beginning of the process; the rate of biodegradation dropped off with the passing of time. As earlier stated, crude and pure refined samples of palm olein, showed high biodegradation rates. With the introduction of TBHQ, weekly oxygen depletion values dropped off rapidly, and the speed of dropping off increased as the antioxidant loading increased. Hence the presence of TBHQ reduced the rate of biodegradation in proportion to its concentration in palm olein.
Using the total cumulative oxygen depletion value of pure refined palm olein at the end of the 28 day period as a standard of comparison, a 0.02% concentration of TBHQ in palm olein resulted in a 25% loss in biodegradability (as compared with the pure sample); a 2% concentration of TBHQ resulted in a 56.5% loss in biodegradability; while a 6% concentration of TBHQ resulted in a 100% loss in biodegradation for palm olein.
The general trend indicated that the presence of the antioxidant brought about a reduction in the biodegradability of vegetable oils. It is clear that TBHQ as an additive retarded biodegradation in proportion to its concentration. Apparently, TBHQ is toxic to microorganisms, and it could be assumed from these preliminary tests that vegetable oils containing TBHQ would be poorly degraded in the environment.
Conclusions
The results of using TBHQ an antioxidant to determine its effect on the biodegradation of palm olein have been presented. Based on the result, the following conclusion may be adduced:
v A 0.02% concentration of TBHQ in palm olein resulted in a 25% loss in biodegradability, 2% concentration of TBHQ resulted in a 56.5% loss in biodegradability and finally, 6% concentration of TBHQ resulted in a total loss in biodegradation for palm olein.
v TBHQ appears to be toxic to microorganisms, and it could be assumed from these preliminary tests that vegetable oils containing TBHQ would be poorly degraded in the environment.
References
1 Nwobi B. E., Ofoegbu O., Adesina O. B., Extraction and Qualitative Assessment of African Sweet Orange Seed Oil., African Journal of Food Agriculture, Nutrition and Development, Volume 6(2), 2006.
2 Bennion M., Introductory Foods.” 10th Edition, Upper Saddle River, New Jersey, USA., Prentice-Hall Inc., 1995.
3 Applewhite T.H., Fats and Fatty Oils. In: Martin Grayson (Executive Editor), Kirk – Othmer Encyclopedia of Chemical Technology, Volume 9. 3rd Edition., New York, John Wiley and Sons Inc., 1978.
4 Masjuki H. H., Maleque M. A., Kubo A., Nonaka T., Palm Oil and Mineral Oil Based Lubricants – their Tribological and Emission Performance., Tribology International, 32 (6):305 – 314, 1999.
5 Honary L. A. T., Biodegradable / Biobased Lubricants and Greases, Machinery Lubrication Magazine, Issue Number 200109, Noria Corporation, www.oilmaintenance.com, 2004.
6 Eastman Chemical Company, Tenox Food-grade Antioxidants for Refined Vegetable Oils, www.eastman.com, Eastman Chemical Company Kingsport Tennessee USA, 2001.
7 Morteza-Semnani K., Saeedi M., Shahani S., Antioxidant Activity of the Methanolic Extracts of Some Species of Phlomis and Stachys on Sunflower Oil, African journal of Biotechnology, 5 (24), 2428 – 2432, 2006.
8 Ferrari R. A., Oliveira V., Scabio A., Oxidative Stability of Biodiesel from Soybean Oil Fatty Acid Ethyl Esters, Sci. Agric. (Piracicaba, Braz.), 62 (3), 291 – 295, 2004.
9 Toshiyuki C., Prediction of Oxidative Stability Based in Various Chemical Properties for Refined Vegetable Oils, Journal of Japan Oil Chemists’ Society, 48 (8), 781-786, 1999.
10 Howell S., Promising Industrial Applications for Soybean Oil in the US, 2007.
11 Ohio State University, Antioxidants, http://class.fst.ohio-state.edu, viewed September 2008.
12 Dunn R. O., Effect of Antioxidants on the Oxidative Stability of Methyl Soyate (Biodiesel), Fuel Processing Technology, 86, 1071 – 1085, 2005.
13 US Army Corps of Engineers, US army Manual EM1110-2-1424 (chapter 8) Viewed from www.usace.army.mil/usace-docs/eng-manuals/em1110-2-1424/c-8.pdf Sept., 2008.
14 www.wiserenewables.com, Wise Solutions-Renewable Lubricants-Biodegradability Primer, (Accessed April 2008).
15 Bioblend “Biodegradable: What does that really mean?” http://www.ignitemediahosting.com/, 2008.
16 Broekhuizen P., Theodori D., Le Blanch K., Ullmer S., Lubrication in Inland and Coastal Water Activities, Tokyo, A. A. Balkema Publishers, 2003.
17 Mecurio P., Burns K. A., Negri A., Testing the ecotoxicology of vegetable versus mineral based lubricating oils: 1. Degradation rates using tropical marine microbes, Environmental Pollution, 129 (2), 165 – 173, 2003.
18 Bloch H. P., Practical Lubrication for Industrial Facilities, The Fairmont Press, Inc., 1999.