Corrosion Inhibition of Mild Steel by Calcium Gluconate in Simulated Cooling Water
Siragul
KARIM1, Chand Mohammad MUSTAFA1, Md.
1 Dept. of Applied Chemistry and
Chemical Technology,
Rajshahi-6205,
2
Dept. of Chemistry,
Rajshahi-6204,
E-mail: mayeedul180@yahoo.com
* Corresponding author: Mayeedul Islam, Tel. +880-1715-234534, Fax. +880-721-750105.
Abstract
Corrosion inhibition of the mild steel by calcium gluconate in simulated cooling water, SCW containing chloride ion was investigated. Electrochemical techniques such as corrosion potential (Ecorr.) measurement and potentiodynamic sweep experiments were used. The experimental parameters were concentration of the inhibitor, pH of the aqueous media and soaking time. Ca-gluconate inhibits mild steel corrosion in near neutral and alkaline (at pH 6 and above) SCW and accelerates corrosion in acidic media (at pH 4 and below). Inhibition activity increases with the increase of gluconate concentration at pH 6 and above and decreases at pH 4 and below. In stagnant SCW, maximum corrosion inhibition was observed at pH 12 for all concentration of Ca-gluconate. Inhibitive action was increased with the increase of soaking time up to six hours and afterwards it remained more or less constant at all concentrations of Ca-gluconate at and above pH 6. Based on the experimental results, mechanisms of action of Ca-gluconate on mild steel corrosion inhibition in SCW have been proposed.
Keywords
Corrosion inhibition; Potentiodynamic sweep; Inhibitor; Ca-gluconate; Simulated cooling water.
Introduction
Although many methods have been employed to prevent the corrosion of steel but the use of inhibitor is the most convenient and economic method in liquid containing system such as in boiler, condenser, heat exchanger, pipe lines, petroleum industries and other chemical process industries [1]. Reports show that there are hundreds of corrosion inhibitors but none of them equals to chromate and dichromate based inhibitor in efficiency [2]. Owing to increasing ecological awareness and strict environmental regulations use of toxic chromate based inhibitors have been restricted or banned [3,4]. Therefore, attention is now focused on the development of substitute nontoxic alternatives to inorganic inhibitors applied earlier.
Gluconates and gluconic acids are known to be cheap, effective, non toxic and environmentally friendly inhibitors for iron and mild steel in cooling water [5,6]. There are reports showing the ability of gluconates to hinder the corrosion of carbon steel and their environmental compatibility [7,8]. Rajendrans et al. [9] reported the inhibition efficiency of Ca-gluconate for mild steel in neutral aqueous solution containing 60 ppm chloride ions. Synergistic inhibition of Ca-gluconate with other substance on mild steel corrosion has also been reported [10]. However, most of the investigations on the inhibition effects of gluconates have been carried out in chloride free or in low chloride neutral aqueous environment in ideal condition [5,10]. These do not reflect the inhibition action in real situation. Simulated cooling water (SCW) (composition 300 ppm Cl-, 351 ppm SO42-, 37 ppm CO32-, and 123.5 ppm HCO3-) is integral part of most of the industries and has effect on the corrosion of materials and scale deposits [11]. Under this perspective the corrosion inhibition action of Ca-gluconate on mild steel has been investigated in realistic aqueous medium like SCW.
Experimental
Commercial grade mild steel coupons (composition: 0.10 C, 0.25 Mn, 0.01 Si, 0.01 P, 0.018 S) having dimension 4 cm × 2 cm × 0.1 cm were used in all experiments. The coupons were polished to mirror finish, degreased with acetone and rinsed with distilled water. The specimens were then insulated with synthetic paint leaving 1 cm × 1 cm area exposed for experiment. These were then dried in open air and stored in a desiccator over silica gel for subsequent use.
The base electrolyte, simulated cooling water (SCW), was prepared by dissolving 500 ppm NaCl, 520 ppm Na2SO4, 170 ppm anhydrous NaHCO3, and 66 ppm Na2CO3 in one liter of distilled water. Inhibitor solution was prepared by dissolving appropriate amount of calcium gluconate in the SCW. The pH of the solution was adjusted by using dilute solutions of NaOH or H2SO4. All reagents used in the study were of analytical grade. Electrochemical cell was composed of platinum counter electrode, prepared mild steel specimen as working electrode and saturated Ag/AgCl (SSE) as a reference electrode. The corrosion potential (Ecorr) was measured against SSE using high impedance (1010 ohm) electrometer. The potentiodynamic experiment was made by using Model: Hab-151 potentiostat at a scan rate of 40 mV/min.
Results
Figure 1 showed the effect of Ca-gluconate concentration on the corrosion potential after 1 hr. immersion. Corrosion potential (Ecorr.) steeply increased with the increase of Ca-gluconate concentration up to 250 ppm, and afterwards levelled off for pH 6 and above. At pH 2 & 4 corrosion potential slowly decreased with the increase of Ca-gluconate concentration. Under identical concentration of Ca-gluconate Ecorr was higher with higher pH. As gluconate is known as an anodic corrosion inhibitor, therefore, increase of corrosion potential is an indication of corrosion inhibition. So, calcium glucoante acted as a corrosion inhibitor at pH 6 and above in stagnant SCW, and aggravated corrosion at or below pH 4.
The effect of soaking time on the corrosion potential of mild steel in stagnant SCW containing Ca-gluconate is shown in Figure 2. The corrosion potential increased slowly with the increase of soaking time up to 6 hours and then it remained constant up to 24 hours immersion (maximum immersion period) for pH 6 and above. For pH 2 and 4 the corrosion potential decreased slowly with the increase of immersion time. This is also an indication of the corrosion inhibition behaviour of calcium gluconate for mild steel in stagnant SCW at pH 6 and above and corrosion aggravation at pH 2 & 4.
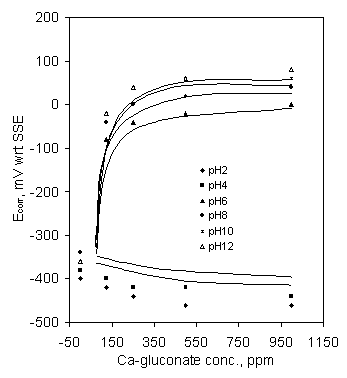
Figure 1. Effect of Ca-gluconate concentration on the corrosion potential of mild steel; soaking time 1 hr
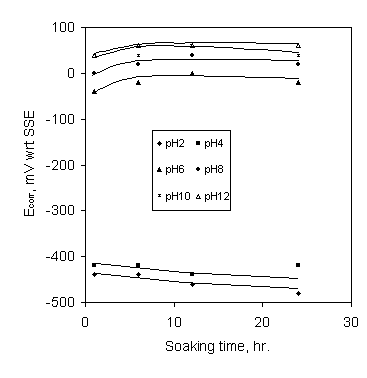
Figure 2. Effect of soaking time on the corrosion potential in SCW containing 250 ppm Ca-gluconate
The potentiodymanic sweep on the mild steel in SCW containing the Ca-gluconate at pH 4 is shown in Figure 3. No current arrest with the increase of potential is an indication of active corrosion. Early current rise at higher concentration of Ca-gluconate shows its corrosion acceleration effect in SCW at pH 4.
Figure 4 shows the potentiodynamic sweep on the mild steel in stagnant SCW containing Ca-gluconate at pH 6. Passivation was observed at all Ca-gluconate concentrations. The extension of passive region was found up to 140mV, 180mV, 200mV and 220mV with respect to SSE in the presence of 125 ppm, 250 ppm, 500 ppm and 1000 ppm Ca-gluconate respectively. The current density having magnitude below 1 mA/cm2 in the current arrest region is an indication of good passivation by Ca-gluconate. In absence of Ca-gluconate, there was no passivation at pH 6.
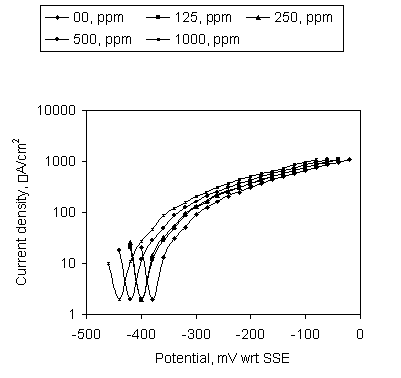
Figure 3. Potentiodynamic sweep on mild steel in SCW containing Ca-gluconate at pH 4; Soaking time 24hr
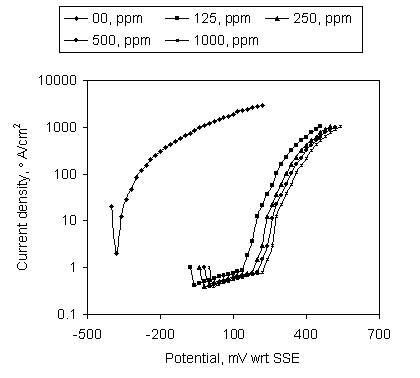
Figure 4. Potentiodynamic sweep on the mild steel in SCW containing Ca-gluconate at pH 6; Soaking time 24hr
Figure 5 shows the effect of pH on the anodic potentiodynamic behaviour of mild steel in SCW containing 250 ppm Ca-gluconate. The extension of the passive range with the increase of pH is observed as the passivation break down potential shifted toward more positive values. These results reveal that Ca-gluconate is a corrosion inhibitor for mild steel in stagnant SCW at pH 6 and above and inhibition activity increases with the increase of Ca-gluconate concentration.
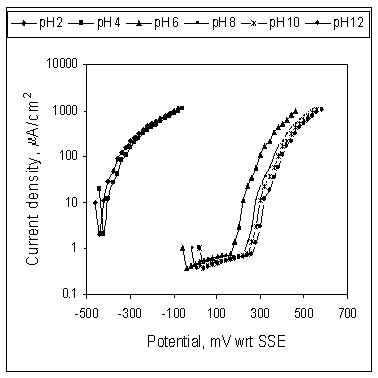
Figure 5. Effect of pH on the anodic potentiodynamic behaviour of mild steel in SCW containing Ca-gluconate; Soaking time 24hr
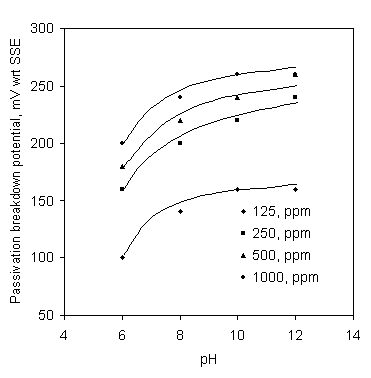
Figure 6. Effect of pH on passivation breakdown potential in SCW containing Ca-gluconate; Soaking time 24 hr
Results for the effect of pH on the passivation breakdown potential of the mild steel in SCW containing Ca-gluconate are shown in Figure 6 for 24 hour soaking. Passivation breakdown potentials are raised to more noble potentials with the increase of pH from 6 to 12. The effect of Ca-gluconate concentration on the passivation breakdown potential of the mild steel is shown in Figure 7. The passivation breakdown potential rapidly increased with the increase of Ca-gluconate concectration.
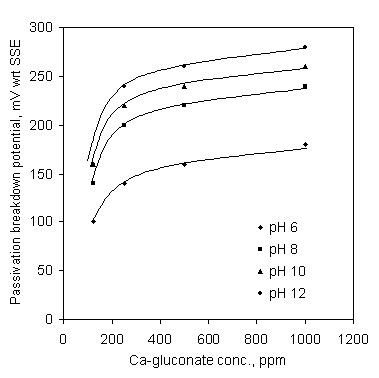
Figure 7. Effect of Ca-gluconate concentration on the passivation breakdown potential in SCW; soaking time 24 hr
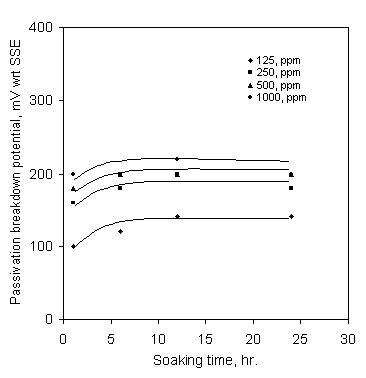
Figure 8. Effect of soaking time on the passivation breakdown potential in SCW containing Ca-gluconate at pH 6
In stagnant condition diffusion is the only process for ions to reach the surface of the mild steel and this is time dependent. As there is always a competition between aggressive ions and Ca-gluconate to breakdown and repair of the surface; therefore, immersion time of the mild steel in stagnant SCW containing Ca-gluconate can have an important role on the corrosion inhibition. The effect of soaking time on the passivation breakdown potential in stagnant SCW containing Ca-gluconate at pH 6 is shown in Figure 8. The breakdown potential slowly increased with the increase of soaking time up to 6 hours and afterwards it remained more or less constant with the further increase of soaking time up to 24 hours for all Ca-gluconate concentrations.
Discussion
In aqueous medium, outer
oxide layer on the mild steel surface becomes hydroxy oxides.
Substitution of
Ca-gluconate dissociates as Ca2+ and gluconate ion in aqueous medium and gluconate forms chelate complex with iron ion. The chelating properties of gluconate could account for its corrosion acceleration in acid media and corrosion inhibition in basic media. Chang and Matijevic have proposed two mechanisms of iron oxide behaviour in the presence of chelating agents: (i) solution coordination mechanism and (ii) surface complexation mechanism [12]. The first mechanism involves reaction of chelating agents with iron (II)-ions, released from the bare oxide surface, accelerating further release of the metal ions from the solids. The second mechanism assumes surface complexation of metal ion with chelating species. In this case, after the formation of surface complex, the relative bond strength of iron ion with the lattice oxygen and with the chelating molecule will be of utmost importance. If the lattice bond between the iron-ion is weakened sufficiently the entire complex will be released into the solution. However, if the bond is stable enough, the complex will stay adsorbed at the surface, where the concentration of the chelating agent in its adsorbed form will play an essential role. It was shown that this process is pH dependent and, in a higher pH environment, iron release could be significantly reduced by formation of Fe (III)-complex that results in progressive development of surface insoluble film.
Surface complexation of iron ion with gluconate ion may be proposed to account for the corrosion inhibition of mild steel in SCW between pH 6 to 12. In this case, gluconate adsorbs on the oxide covered steel surface forming an iron-gluconate surface complex with the Fe ion on top of the oxide layer as given below-
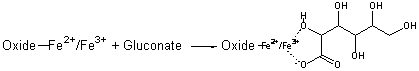
This adsorbed layer blocks the adsorption of Cl- ion on the oxide surface preventing oxide dissolution through soluble FeOCl formation. In this way Ca-gluconate inhibit mild steel corrosion if the oxide layer is present on the surface. Similar formation of the adsorbed complex of ascorbate on the iron oxide surface has been reported [13].
In presence of gluconate in SCW, a competition starts between Cl- and gluconate to reach the surface. In stagnant condition, gluconate and Cl- ions can only reach the mild steel surface by diffusion. So, few times are required for the accumulation of sufficient amount of bigger sized gluconate on the surface to produce complete barrier against chloride ion.
Oxide layer can not exist on the steel surface below pH 4 [14]. In absence of oxide layer, iron surface becomes bare and freely corrodes as-
Fe+2H+ ® Fe2++H2
When Ca-gluconate is added to the acidic SCW, Fe(gluconate)2 chelating complex of very high solubility easily forms with Fe2+ and there by accelerating corrosion. Similar mechanism of corrosion acceleration of mild steel by ascorbic acid in acid media has been suggested [13].
Conclusions
1. Ca-gluconate can be used as an effective and environmental friendly corrosion inhibitor for mild steel in SCW.
2. It inhibits mild steel corrosion in near neutral and alkaline media ( at pH 6 and above) and accelerates in acid media (at pH 4 and below).
3. The inhibition activity increases with the increase of both Ca-gluconate concentration and pH.
4. Inhibitive action of Ca-gluconate is assumed due to the formation of adsorption complex with iron ions in the oxide of the surface.
5. The presence of oxide layer on the mild steel surface is essential for the corrosion inhibition by gluconate in SCW.
References
1. Ayo Samuel AFOLABI, Synergistic inhibition of potassium chromate and sodium nitrite on mild steel in chloride and sulphide media, Leonardo Electronic Journal of Practices and Technologies, 2007, 11, p. 143-154.
2. Ali M. R., and Mustafa C. M., Effect of molybdate, nitrite and zinc ions on the corrosion inhibition of mild steel in aqueous chloride media containing cupric ions, J. Sci. Res., 2008, 1, p. 82-91.
3. Mustafa C. M., and Shahinur Islam Dulal S. M., Molybdate and Nitrite as corrosion inhibitors for copper-coupled steel in simulated cooling water, Corrosion Science, 1996, 52, p. 16-22.
4. Lehman A. J., and Laug E. P., Procedures for the appraisal of the toxicity of chemicals in foods, Food Drug Cosmet. Law J., 1955, 10, p. 679-748.
5. Shibli S. M. A., and Anitha Kumary V., Inhibitive effect of cacium gluconate sodium molybdate on carbon steel, Anti-Corrosion Methods and Materials, 2004, 51, p. 277-281.
6. Sing I. B., Venkatachari G., and Balakrishnan K., Inhibition effect of sodium boro-gluconate on mild steel with and without nitrite ions in low chloride containing water, Journal of Applied Electrochemistry, 1993, 24, p. 179-183.
7. Lahodny-Sarc, Kapor O. F., and Halle R., Corrosion inhibition of carbon steel in chloride solutions by blends of calcium gluconate and sodium benzoate, Materials and Corrosion, 2000, 51, p. 147-151.
8. Lahodny-Šarc, Olga Kapor Frankica and Halle Radovan, Green corrosion inhibitors, European Coatings, 2000, 76, p. 51-53.
9. Rajendran S., Apparao B. V., and Palaniswamy N., Calcium gluconate as corrosion inhibitor for mild steel in low chloride media, British Corrosion Journal, 1998, 33, p. 315-317.
10. Gunasekaran G., Planiswamy N., Apparao B. V., and Muralidharan V. S., Enhanced synergistic inhibition by calcium gluconate in low chloride media. Part I. Kinetics of corrosion, Proc. Indian Acad. Sci. (Chem. Sci.), 1996, 108, p. 399-405.
11. Farooqi I. H., Quraishi M. A., and Saini P. A., Recent Trends in Cooling Water Inhibitors, CORROSION 2000, Paper Number 00332, NACE International, Aligarh Muslim University, Orlando.
12. Chang H.-C., and Matijevic B., Interactions of metal hydrous oxides with chelating agents: IV. Dissolution of hematite. J. Colloid Interface Sci., 1983, 92, p. 479-488.
13. Valek L., Matinez S., Mikulic D. and Brnardic I., The inhibition activity of ascorbic acid towards corrosion of steel in alkaline media containing chloride ions, Corrosion Science, 2008, 53, p. 2705-2709.
14. Uhlig H. H., and Revie R. W., Corrosion and Corrosion Control, 3rd. ed, John Wiley & Sons, New York, 1991.