Effects of Alumina Cement on the Refractory Properties of Leached Ipetumodu Clay
Davies Oladayo FOLORUNSO1,2*, Peter Apata OLUBAMBI2,3,
and Joseph Olatunde BORODE 1,2
1 Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria
2Africa Materials Science And Engineering Networks: A Carnegie-IAS (RISE) Networks
3Department of Chemical and Metallurgical Engineering, Tshwane University of Technology, Pretoria West, Pretoria, South Africa.
E-mails: stdavies4ever@yahoo.com, polubambi@yahoo.com, borode202@yahoo.co.uk
* Corresponding author: Phone: +2348038204252
Abstract
The effect of alumina cement (Fe2O3) on the refractory properties of leached Ipetumodu clay has been studied. The raw clay was analysed using Scanning Electron Microscope (XL 30 ESEM/EDX), X-Ray Diffraction Machine (Philips PW 3710 with PW 1752 graphite monocromator) and X-Ray Fluorescence Machine (ARL 8410) in order to determine the purity level. The tests revealed an average of 5.7% Fe2O3 in the clay. The clay was then purified hydrometallurgical using different concentrations of oxalic acid (0.4, 0.8, 1.2, 1.6 and 2.0 mol/dm3) and combination of different times (30, 60, 90, 120 and 150 min), temperatures (30, 50, 70 and 90ºC) and agitation speeds (120, 160, 200 and 240 rev/min). The purification process as revealed by Atomic Absorption Spectrophotometry showed that Fe2O3 was reduced to 0.96%. Samples of leached clay containing different quantities of alumina cement, silica sand and sawdust were prepared, dried at 110ºC for 24 hours and fired at 900, 1100, 1300 and 1500ºC at rate of 4ºC /min, soaked for 2 hrs. These samples were presented for refractory tests (permanent linear change, refractoriness under load, thermal shock resistance, modulus of rupture, bulk density, cold crushing strength and apparent porosity). For all the properties tested, 3% sawdust, 20% silica sand and 10% alumina addition gave the optimum result with reliable phase integrity, as revealed by scanning electron microscopy.
Keywords
Hydrometallurgy; Leaching; Diffraction; Spectrophotometry; Purification; Refractoriness; Phase Integrity and Thermal Shock.
Introduction
Clay is a general term including many combinations of one or more clay minerals with traces of metal oxides and organic matter [1]. Geologic clay deposits are mostly composed of phyllosilicate minerals containing variable amounts of water trapped in the mineral structure.
It is a hydrous aluminosilicate (Al2O3•2- SiO2•2H2O) [2], and an essential resource in porcelain and ceramic manufacturing [3,4], production of paper, pigments, and fillers. It is formed by the mechanical and chemical breakdown of rocks. Depending on the atmospheric and geological condition of deposition, as well as the degree of alteration of the clay, iron (hydr)oxides (usually Fe3+ forms) are commonly precipitated or adsorbed to the clay surfaces or admixed as a separate phase [5,6] that make much of the clay unusable for commercial application due to insufficient whiteness [4], and the reduction of refractoriness of products [7]. So, for the reasons mentioned above, the quality of clay is measured in terms of iron content [7].
Some researchers have developed different physical and chemical techniques (and recently microbiological) with the purpose of removing the ferric iron present as oxide or hydrated oxide in the clay. These techniques generally include magnetic separation, froth flotation, selective flocculation, size separation by hydrocyclones, and leaching [4,7,8]. The leaching technique was however employed in this work.
Alumina cements are materials consisting predominantly of hydraulic calcium aluminates. Alternative names are "aluminous cement", "high-alumina cement" and "Ciment fondu" in French. They are used in a number of small-scale, specialized applications [1].
The main active constituent of calcium aluminate cements is monocalcium aluminate (CaAl2O4, CaO Al2O3, or CA in the cement chemist notation). It usually contains other calcium aluminates as well as a number of less reactive phases deriving from impurities in the raw materials. Rather a wide range of compositions is encountered, depending on the application and the purity of aluminium source used [9].
Refractory materials are heat-resistant compounds that can withstand the corrosive and abrasive effects of liquids and gases without contaminating other materials. Refractory materials are essential in metallurgy and heavy industry for their durability under extreme heat. Refractories generally consist of materials with high melting temperatures which are chemically bonded to create compounds that can withstand temperatures over 1550ºC (the melting point of iron). Refractory materials typically consist of oxides of silicon, aluminium, magnesium, calcium and/or zirconium. Refractory materials are divided into classes according to their chemical composition: acidic, neutral and basic. Silicon and zirconium refractories are acidic. Alumina, chrome and carbon refractories are neutral. Magnesia, dolomite and chrome magnesite refractories are basic.
With the background knowledge of what clays, alumina cement and refractory materials are, it is therefore possible to formulate an appropriate refractory mix that will work to a reasonably appreciable standard. This work is aimed, therefore, to exploit the possibility of utilizing the indigenous clay for the production of insulating refractory for use in the industries.
Material and Method
The materials used in the research work are clay from a deposit in Ipetumodu in Osun State, Nigeria, secar 71 alumina cement, 99.8 % purity oxalic acid and deionized water.
Preparation of the Raw Clay for Analyses
The clays, after being mined from the deposit was washed in water and the deleterious particles in it was removed by decantation. It was then drained in Plaster of Paris (P.O.P.) mould for effective removal of the water molecules contained there-in, sun-dried for three days and later in the oven at a temperature of 90 0C for 8 hours. The dried clay was then crushed in a jaw crusher, ground in a Rawley Sussex grinder and subsequently sieved to finer size of 5.50 µm.
Mineralogical Characterization of the Raw Clay
The Scanning Electron Microscopy/Energy Dispersive Spectroscopy (SEM/EDS), X-Ray Diffraction (XRD) and X-Ray Fluorescence (XRF) of the ground clay in the raw state were done using Scanning Electron Microscope (XL 30 ESEM/EDX), X-Ray Diffraction Machine (Philips PW 3710 with PW 1752 graphite monocromator) and X-Ray Fluorescence Machine (Model ARL 8410) in order to determine the morphology of the clay, the phase identification and the relative proportions of the constituent elements respectively. These tests revealed that the clay deposit contained an average of 5.652% of the most deleterious of the impurities (Fe2O3).
Purification Using Conventional Hydrometallurgical Techniques
The clay was prepared for hydrometallurgical purification using oxalic acid of different concentrations (0.4, 0.8, 1.2, 1.6 and 2.0 mol/dm3) at different times (30, 60, 90, 120 and 150 minutes), temperatures (30, 50, 70 and 90ºC) and agitation speeds (120, 160, 200 and 240 rev/min). For iron oxide removal, oxalic acid is five times more effective than inorganic acids, and is capable of complexing and reducing iron [10]. In order to dissolve a mole of iron, three equivalents of oxalic acid are necessary [11].
|
3C2O42- + Fe3+ = [Fe (C2O4)3]3- |
(1) |
The results of the purification processes revealed that the optimum conditions of purification for the deposit is 1.6 mol/dm3 at 90ºC for 150 mins and 200 rev/min. Bulk of the clay was then purified at the identified optimum conditions of purification and again analysed using the XRF, XRD and SEM/EDS in order to confirm the extent of Fe2O3 removal. The results revealed that Fe2O3 was reduced by 80.61%.
Determination of the Refractory Properties of Purified Sample
The purified clay with varying quantities (5, 10, 15, 20, 25, 30, 35 and 40%) of high alumina cement (Secar 71) was formed into cylindrical (50mm diameter x 50 mm high) specimens after mixing with about (10-15)% deionized water. The samples were dried in air for 24 hrs and later in the oven at 110ºC for 48 hrs. They were subsequently tested in accordance to the American Standard for Testing and Materials (ASTM) for the following properties:
(a) Permanent linear change
Certain permanent changes occur in materials during heating and these changes may be due to change in the allotropic form, chemical reaction, and liquid phase formative sintering reactions. These changes determine the volume stability, expansion and shrinkage of the refractory at high temperatures. The samples’ heights were measured before and after firing at various temperatures (900°C, 1100°C, 1300°C and 1500°C) with the vernier calliper at three different points and the average taken. The changes in the heights were then measured and calculated as the percentage permanent linear change for each of the samples.
(b) Modulus of rupture (MOR)
The modulus of rupture is defined as the maximum stress a rectangular test piece (150 mm × 25 mm × 25 mm) can withstand in a 3-point bending test until it breaks. It is expressed in N/mm2 or MPa (ISO 5013). Samples were prepared in the required proportions, dried at 110°C and fired to 1500°C. Some of the samples were rupture tested after cooling to the room temperature while the remaining was tested at the elevated temperature of 1500°C. Five samples were tested at each of the temperatures and the average computed in order to get more reliable results.
(c) Thermal shock resistance (TSR)
Test samples were put in the furnace that was maintained at 1100°C and soaked at that temperature for 30 mins. They were then removed from the furnace with crucible tongs and cooled in air for 10min. The test samples were examined to note the presence of cracks and then returned into the furnace, heated for 10mins and cooled outside again for 10mins. This cycle of heating and cooling was repeated for a number of times until fracture occurred (ASTM C133). This number of complete cycles to produce failure on each sample was noted and taken as the measure of the thermal shock resistance.
(d) Refractoriness under load (RUL)
Refractoriness under load, according to International Standard Organization [14] is a measure of the deformation behaviour of refractory ceramic products subjected to a constant load (0.2 Nmm-2) and increasing temperature. Samples with dimension 50 mm high by 50 mm diameter were dried at 110°C for 48 hours and after cooling to the room temperature, were drilled co-axially with holes of 12.5 mm diameter. They were then set in the RUL furnace one after the other and the temperature at which the height of each of the samples changed by 0.5 % noted and recorded as the refractoriness of the samples under load.
(e) Bulk densities and Cold crushing strengths
The sizes (heights and diameters), weights and volumes of samples dried at 110°C were measured in order to compute the bulk densities. The same thing was done to samples fired at 900°C, 1100°C, 1300°C and 1500°C after cooling to the room temperature. These fired samples were also put under the Auto Compression Testing Machine (Pat 2001) in order to determine the loads under which they crumble. These loads under which they just began to crack represent their cold crushing strengths.
(f) Apparent porosity
The apparent porosity is the volume of the open pores, into which a liquid can penetrate, as a percentage of the total volume of the refractory. Apparent porosities were measured without and with 1-5% sawdust to determine the optimum quantity of sawdust required to produce the averagely required value for the porosity (45 -70%) of good insulating firebricks [12].
Results and Discussion
Figure 1 depicts the relative proportions of the various components of the clay. The spectral reveals that silica (SiO2) and alumina (Al2O3) are the major desirable components of the clay, while the oxides of iron, manganese, sodium, potassium, chromium, vanadium, zirconium and titanium are the deleterious ones. However, only the oxide of iron is the most detrimental to good performance of the clay for refractory applications, just as the remaining deleterious oxides are present in such negligible proportions that their presence would not constitute any noticeable threats to good performance of the clay for the desired applications. This therefore forms the basis for paying special attention to the removal of the oxide of iron by leaching technique.
Table 1 further corroborates the revelations of the X-ray diffraction analysis by giving the quantitative relative abundance of the various compounds present in the clay. The table shows that SiO2 and Al2O3 which are the desirable compounds are in the largest proportions while Fe2O3 and the other deleterious compounds are as shown. As a result of the purification exercise, the percentage abundance of Fe2O3 reduced from 4.95 to 0.96 %, the relative abundance of CaO increased from 0.53 to 3.78 %, the combination of Na2O, MgO and K2O, after leaching reduced from 4.49 to 1.38 % which is all expected attributes of a good refractory material [13].
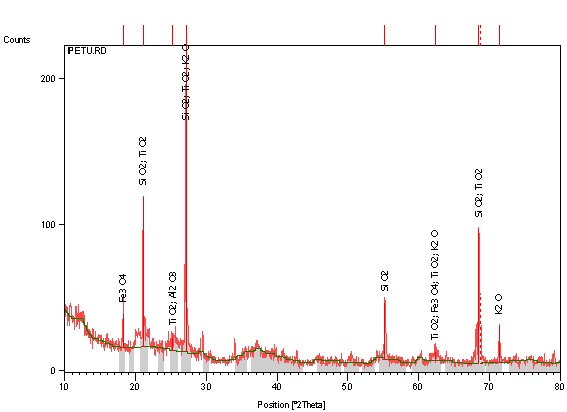
Figure 1. X-ray diffraction pattern of Ipetumodu clay
Table 1. XRF semi-quantitative analysis of the elements in raw and purified Ipetumodu clay
|
Compound |
SiO2 |
Al2O3 |
Fe2O3 |
K2O |
CaO |
TiO2 |
MnO |
Total |
|
Raw Clay |
59.76 |
21.98 |
4.95 |
2.90 |
0.53 |
0.86 |
0.08 |
|
|
Leached Clay |
59.05 |
25.74 |
0.96 |
1.18 |
3.78 |
1.88 |
0.03 |
|
|
Compound |
MgO |
Na2O |
P2O5 |
Cr2O3 |
V2O5 |
ZrO2 |
LOI |
|
|
Raw Clay |
0.61 |
0.98 |
0.05 |
0.01 |
0.01 |
0.08 |
7.89 |
100.69 |
|
Leached Clay |
0.12 |
0.08 |
0.05 |
0.02 |
0.02 |
0.04 |
7.31 |
100.26 |
The Scanning Electron Microscopy (figure 2) reveals the morphology of the clay and the diffraction spectra. The EDX indicates the quantity of iron oxide (6.45 %) in the clay which is a further confirmation of the reliability of the XRD analysis.
Figure 3 shows the variation of the cold crushing strengths with the quantity of silica sand added to the leached clay. The figure revealed that 20 % silica content exhibited the optimum strength, which therefore formed the basis for choosing 20 % silica sand content for subsequent refractory tests. Figure 4 revealed that the leached clay exhibited greater strengths upon the addition of alumina than the raw clay and that the strengths increased progressively with the addition of alumina.
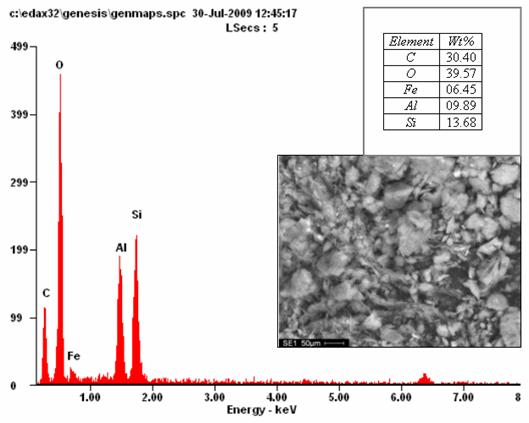
Figure 2. Typical SEM/EDX of Ipetumodu clay showing the morphology of the clay and its chemical composition (x500)
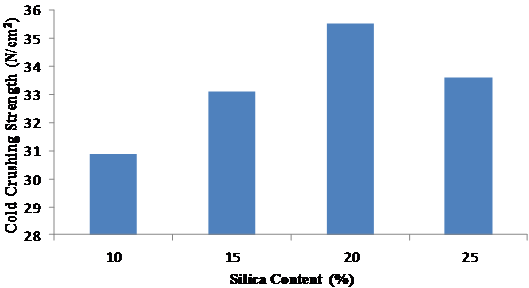
Figure 3. Variation of Cold Crushing Strengths with Silica Content
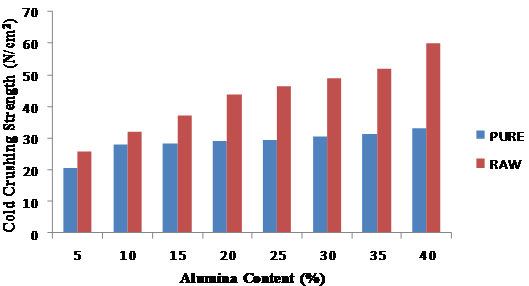
Figure 4. Variation of Cold Crushing Strengths with Alumina Content
Figure 5 shows how the crushing strengths vary with alumina content and firing temperatures. The figure reveales that the mix could not withstand temperature as high as 1500°C when the alumina content was above 20 %. It was also clearly observed that 10 % alumina exhibited the greatest potential for refractory application because it was the one that possessed the highest crushing strength at the highest firing temperature of 1500°C.
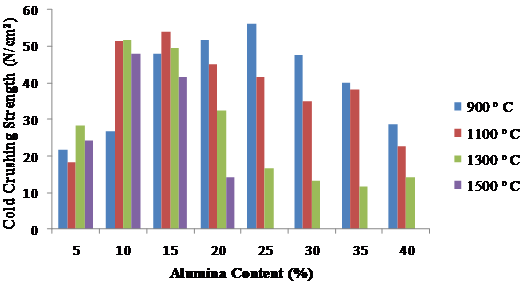
Figure 5. Variation of cold crushing strengths with alumina at various firing temperatures
Figure 6 depicts the various expansions and contractions of the leached clay upon the addition of different quantities of alumina cement. The expansions and contractions are however within the limits of ±0.4 % allowed for good refractory materials (ASTM C 210).
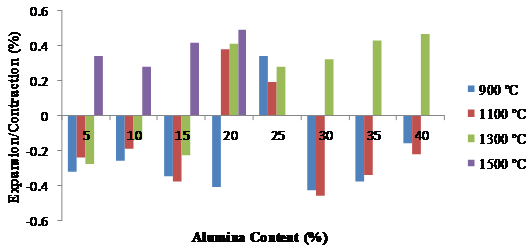
Figure 6. Variation of thermal linear expansions with alumina at different firing temperatures
The modulus of rupture increased progressively with alumina content when the samples were tested upon cooling to room temperature (Figure 7). When tested at the elevated temperature of 1500ºC, 10 % alumina content exhibited the most promising result of about 27.35 N/cm2. The clay is therefore a good refractory material since the standard value of modulus of rupture for good refractory materials is in the range 14 – 31 N/cm2 (ASTM C133).
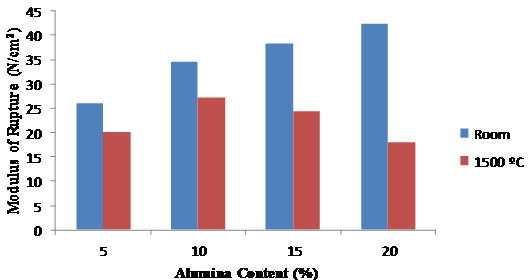
Figure 7. Modulus of rupture at room temperature and 1500 ºC
Figure 8 shows that 5 and 10 % alumina content exhibited the best values for thermal shock resistance since the acceptable range is 15 -30 cycles (ASTM C201).
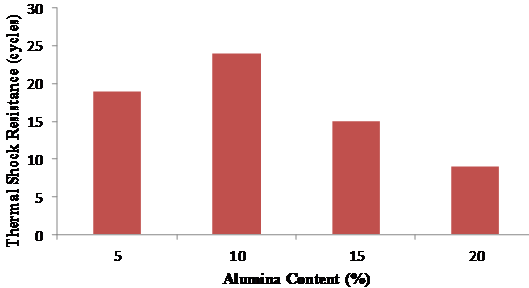
Figure 8. Thermal shock resistance at various alumina contents
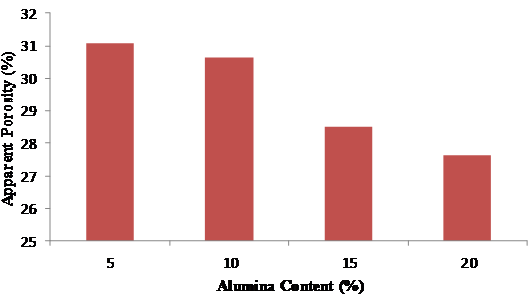
Figure 9. Variation of apparent porosity with alumina content
The apparent porosity decreases with alumina content (Figure 9) as a result of the binding effect of the alumina cement which effectively reduces the pores in the mix. However, on the introduction of sawdust in varying proportions, the apparent porosity increases with the quantity of sawdust (Figure 10). The later trend is attributed to the fact that the sawdust burnt off at the elevated temperature of 1500ºC thereby created pores which is proportional to the quantity of sawdust added. The more the quantity of sawdust added the more the pores created after firing.
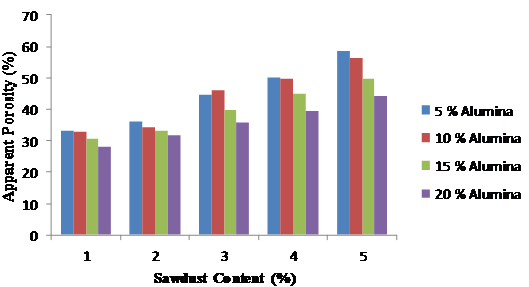
Figure 10. Variation of apparent porosity with sawdust
The refractoriness under load (figure 11) is maxim at 1230ºC for 10 % alumina addition, followed by 5 % alumina at 1050ºC. The clay will therefore work well under load, up to about 1230 ºC.
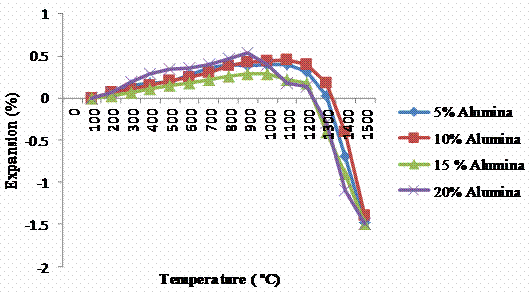
Figure 11. The refractoriness under load at different alumina contents
Conclusion(s)
The results obtained have shown that:
1. The combination of the four characterization techniques adopted in this study (XRD, XRF, AAS & SEM/EDS) showed consistency in the relative quantities of the desired elements (silica, alumina and lime) and the deleterious impurities (oxides of Fe, K, Mn, Mg and Na) contained in the clays;
2. The application of oxalic acid in the purification exercise revealed that the optimum conditions of purification of the clay is 1.6 mol/dm3 of oxalic acid at 90ºC for 150 min and 200 rev/min;
3. The addition of alumina cement, silica sand and sawdust in the appropriate proportions enhanced the refractory properties of the purified clay;
4. Leached Ipetumodu clay with the addition of 10% alumina, 20% silica and 3% sawdust exhibited the greatest potential for the production of insulating firebricks because it was able to maintain compositional and phase stability at a very high temperature of 1500ºC.
Acknowledgements
The authors wish to express their profound gratitude to the following bodies for their support for the research work: Federal University of Technology, Akure (F.U.T.A.), Africa Materials Science and Engineering Network (AMSEN), Regional Initiatives in Science and Education(RISE), Science Initiative Group (SIG), Vesuvius South Africa, Tshwane University of Technology (TUT), South Africa.
References
1. Balek V., Perez Rodriguez J.L., Pérez-Maqueda L.A., Šubrt J., Poyato J., Thermal behaviour of ground vermiculite, Journal of Thermal Analysis and Calorimetry, 2007, 88(3), p. 819-823.
2. Al-Ani T., Sarapää O., Lehtinen M.J., Mineralogical and chemical study of some kaolin samples from the Kahdeksaisiensuo and Hyväjärvi occurrences, Virtasalmi SE Finland Geologian tutkimuskeskus, 2006, Available at: http://arkisto.gtk.fi/m19/3232/M19 _3232_2006_1_82.pdf.
3. Asmatulu R., Removal of discolouring contaminants of east Georgia kaolin clay and its dewatering, Turk. J. Eng. Environ. Sci., 2002, 26, p. 447-453.
4. Ruiz C., Genesis and evolution of the kaolin-group minerals during the diagenesis and the beginning of metamorphism, Department of Inorganic Chemistry, mineralogy and crystallography. Faculty of Sciences. University of Malaga. 29071 Malaga (Spain), 2006.
5. Mosser-Ruck R., Devineau K., Charpentier D., Cathelineau M., Effects of ethylene glycol saturation protocols on XRD patterns: A critical review and discussion, Clays and Clay Minerals, 2005, 53(6), p. 631-638.
6. Reeves G.M, Sims I., Cripps J.C. (Eds). Formation and alteration of clay materials; Geological Society, London, Engineering Geology Special Publications, 2006, 21, p. 29-71.
7. Murray H.H.,. Applied Clay Mineralogy: Occurrences, Processing and Applications of Kaolins, Bentonites, Palygorskite -Sepiolite and common clays, Pub. By Elsevier, 2007
8. Lee E.Y., Cho K.S., Ryu H.W, Microbial refinement of kaolin by iron-reducing bacteria. Appl. Clay Sci., 2002, 22, p. 47-53.
9. Hillier S., Clay Mineralogy, pp 139-142 In: Middleton G.V., Church M.J., Coniglio M., Hardie L.A. and Longstaffe F.J.(Editors) Encyclopaedia of sediments and sedimentary rocks. Kluwer Academic Publishers, Dordrecht, 2003.
10. Taylor H.F.W., Cement Chemistry, Academic Press, ISBN 0-12-683900-X, p. 317, 1990.
11. Mulligan C.N., Kamali M., Gibbs B.F., Bioleaching of heavy metals from a low-grade mining ore using Aspergillus niger. J. Hazard Mater., 2004, 110, p. 77-84.
12. Azom T., Refractories-An overview, Journal of Materials 2008, Available at: www.azom.com, (accessed 04/01/2011)
13. Folorunso D.O., Aribo S., Olubambi P.A., Borode, J.O., Hydrometallurgical Purification of Some Clay Deposits for High Temperature Applications, Journal of Minerals and Materials Characterization and Engineering, 2012, 11(5), p. 461-469.
14. ISO 1893, Refractory products -- Determination of refractoriness-under-load (differential - with rising temperature)