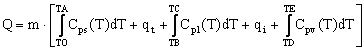Thermal Energy Storage with Phase Change Material
Lavinia Gabriela SOCACIU
Department of Mechanical Engineering, Technical University of Cluj-Napoca, Romania
E-mail: lavinia.socaciu@termo.utcluj.ro
* Corresponding author: Phone: +40744513609
Abstract
Thermal energy storage (TES) systems provide several alternatives for efficient energy use and conservation. Phase change materials (PCMs) for TES are materials supplying thermal regulation at particular phase change temperatures by absorbing and emitting the heat of the medium. TES in general and PCMs in particular, have been a main topic in research for the last 30 years, but although the information is quantitatively enormous, it is also spread widely in the literature, and difficult to find. PCMs absorb energy during the heating process as phase change takes place and release energy to the environment in the phase change range during a reverse cooling process. PCMs possesses the ability of latent thermal energy change their state with a certain temperature. PCMs for TES are generally solid-liquid phase change materials and therefore they need encapsulation. TES systems using PCMs as a storage medium offers advantages such as high TES capacity, small unit size and isothermal behaviour during charging and discharging when compared to the sensible TES.
Keywords
Phase Change Material (PCM); Thermal Energy Storage (TES).
Introduction
Thermal energy storage (TES) is defined as the temporary holding of thermal energy in the form of hot or cold substances for later utilization [1]. Energy demands vary on daily, weekly and seasonal bases. These demands can be matched with the help of TES systems that operate synergistically, and deals with the storage of energy by cooling, heating, melting, solidifying or vaporizing a material and the thermal energy becomes available when the process is reversed. TES is a significant technology in systems involving renewable energies as well as other energy resources as it can make their operation more efficient, particularly by bridging the period between periods when energy is harvested and periods when it is needed. That is, TES is helpful for balancing between the supply and demand of energy [1,2].
TES systems have the potential for increasing the effective use of thermal energy equipment and for facilitating large-scale fuel commutating [2]. The selection of a TES system for a particular application depends on many factors, including storage duration, economics, supply and utilization temperature requirements, storage capacity, heat losses and available space [3].
The main types of TES are sensible and latent. Sensible TES systems store energy by changing the temperature of the storage medium, which can be water, brine, rock, soil, etc. Latent TES systems store energy through phase change, e.g., cold storage water/ice and heat storage by melting paraffin waxes. Latent TES units are generally smaller than sensible storage units. More compact TES can be achieved based on storages that utilize chemical reactions [1].
A complete TES process involves at least three steps: charging, storing and discharging. In practical systems some of the steps may occur simultaneously (for example charging and storing) and each step may occur more than once in each storage cycle. In figure 1 is illustrated a simple storage cycle, in which the three steps are shown distinct. Where the heat Ql is infiltrating and is positive in value for a cold thermal storage. If it is released, it will be toward the surrounding and Ql will be negative. The heat flow is illustrated for the storing process, but can occur in all three processes [3].
In figure 2 is presented the increase of internal energy, when energy in the form of heat is added to a substance. The well-known consequence is an increase in temperature (sensible TES) or change of phase (latent TES). Starting with an initial solid state at point O, a heat addition to the substance first causes sensible heating of the solid (region O–A), followed by a solid-to-liquid phase change (region A–B), a sensible heating of the liquid (region B–C), a liquid-to-vapour phase change (region C–D), and a sensible heating of the vapour (region D–E). The total amount of heat can be written in the following formula [4]:
|
|
(1) |
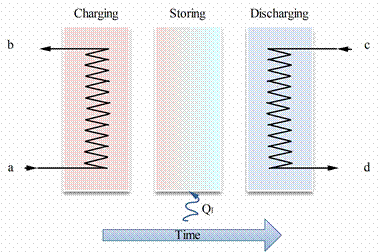
Figure 1. The three processes in a general TES system
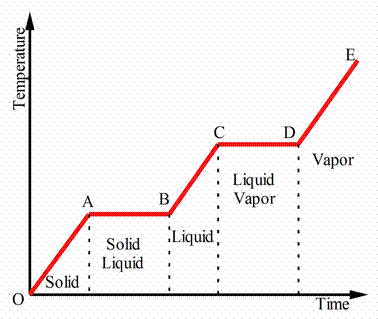
Figure 2. Temperature-time diagram for the heating of a substance
Latent heat storage is one of the most efficient ways of storing thermal energy [5]. In latent TES systems, energy is stored during the phase change (e.g. melting, evaporating and crystallization). Due to the specific heat of a typical medium and the high enthalpy change during phase change, the latent heat change is usually greater than the sensible heat change for a given system size [1]. Unlike the sensible heat storage method, the latent heat storage method provides much higher storage density, with a smaller temperature difference between storing and releasing heat. Every material absorbs heat during heating process while its temperature is rising constantly. The heat stored in the material is released into the environment through a reverse cooling process. During the cooling process, the material temperature decreases continuously [5].
The stored energy during a latent storage process can be evaluated as:
|
Q=m·L |
(2) |
where m denotes the mass and L is the specific latent heat of the PCM (Phase Change Material) [1].
Latent TES systems store energy in PCMs, with the thermal energy stored when the material changes phase, usually from a solid to liquid (for example: energy is required to convert ice to water, to change water to steam and to melt paraffin wax).
The most common example of latent TES is the conversion of water to ice. Cooling systems incorporating ice storage have a distinct size advantage over equivalent-capacity chilled-water units became of the relatively large amount of energy that is stored through the phase change [3]. For minus (cold) temperature, PCMs (i.e. ice), the liquid to solid (freezing) change absorbs energy and the solid to liquid change releases that absorbed energy. On the other hand, for positive (hot) temperature PCMs, the solid to liquid change absorbs energy and the liquid to solid change releases that absorbed energy, and does so at constant temperatures. In each case, the amount of energy absorbed and released is termed as latent heat [6].
Phase change process of PCM from solid to liquid and vice versa is schematically shown in figure 3.

Figure 3. Schematic representation of phase change process
The large heat transfer during the melting process as well as the crystallization process without significant temperature change makes PCM interesting as a source of heat storage material in practical applications. When temperature increases, the PCM microcapsules absorbed heat and storing this energy in the liquefied phase change materials. When the temperature falls, the PCM microcapsules release this stored heat energy and consequently PCM solidify [5].
The energy required to cause these changes is named the heat of fusion at the melting point and the heat of vaporization at the boiling point. The specific heat of fusion or vaporization and the temperature at which the phase change occurs are very important in design phase.
PCMs are either packaged in specialized containers such as: tubes, shallow panels, plastic bags; or contained in conventional building elements such as: wall board and ceiling; or encapsulated as self-contained elements [1,3].
The aim of this research paper was to provide a compilation of practical information on different PCMs and systems developed for thermal management in residential and commercial establishments based on TES technology in building integrated energy system.
Material and Method
Types of PCM
Figure 4 illustrated a classification of PCMs, but generally speaking PCMs can be broadly classified into two types: Organic PCMs e.g. Paraffin Wax and Inorganic PCMs e.g. Salt Hydrates [7-9].
Early efforts in the development of latent TES materials used inorganic PCMs. These materials are salt hydrates, including Glauber’s salt (sodium sulphate decahydrate), which was studied extensively in the early stages of research into PCMs [10,11].
The phase change properties of inorganic PCMs are shown in table 1 [9-12] and the most promising selection of organic PCMs is shown in table 2 [10,11].
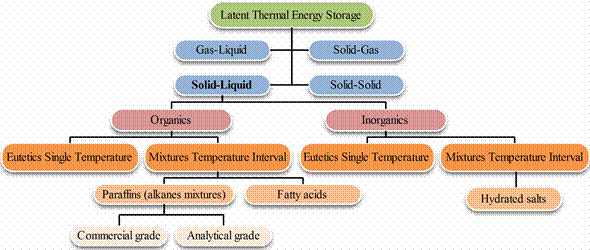
Figure 4. Classification of PCMs
Table 1. Inorganic PCMs (Typical Values)
|
PCM Name |
Melting Temperature [ºC] |
Heat of Fusion [kJ/kg] |
Thermal conductivity [W/mK] |
Density [kg/m3] |
|
KF·4H2O Potassium fluoride tetrahydrate |
18.5 |
231 |
n.a. |
1447 (liquid,20ºC) 1455 (solid, 18ºC) |
|
Mn(NO3)2·6H2O Manganese nitrate hexahydrate |
25.8 |
125.9 |
n.a. |
1738 (liquid,20ºC) 1728 (liquid, 40ºC) 1795 (solid, 5ºC) |
|
CaCl2·6H2O Calcium chloride hexahydrate |
29.0 |
190.8 |
0.540 (liquid, 38.7ºC) 1.088 (solid, 23ºC) |
1562 (liquid, 32ºC) 1802 (solid, 24ºC) 1710 (solid, 25ºC) |
|
CaBr2·6H2O Calcium bromide hexahydrate |
34 |
115.5 |
n.a. |
1956 (liquid, 35ºC) 2194 (solid, 24ºC) |
|
Na2SO4·10H2O Sodium sulphate decahydrate |
32.4 |
254 |
0.544 |
1485 (solid) |
|
Na2CO3·10H2O Sodium carbonate decahydrate |
34.2 |
246.5 |
n.a. |
1442 |
|
Na2HPO4·12H2O Sodium orthophosphate dodecahydrate |
35.5 |
265 |
n.a. |
1522 |
|
Zn(NO3)2·6H2O Zinc nitrate hexahydrate |
36.2 |
146.9 |
0.464 (liquid, 39.9ºC) 0.469 (liquid, 61.2ºC) |
1828 (liquid, 36ºC) 1937 (solid, 24ºC) 2065 (solid, 14ºC) |
|
n.a.=not available |
||||
Table 2. Organic PCMs (typical values)
|
PCM Name |
Melting Temperature [ºC] |
Heat of Fusion [kJ/kg] |
|
|
CH3(CH2)16COO(CH2)3CH3 |
Butyl stearate |
19 |
140 |
|
CH3(CH2)11OH |
l-dodecanol |
26 |
200 |
|
CH3(CH2)12OH |
l-tetradecanol |
38 |
205 |
|
CH3(CH2)n(CH3… |
Paraffin |
20-60 |
200 |
|
45%CH3(CH2)8COOH 55%CH3(CH2)10COOH |
45/55 capric-lauric acid |
21 |
143 |
|
CH3(CH2)12COOC3H7 |
Propyl palminate |
19 |
186 |
PCMs Properties
Inorganic PCMs have some attractive properties including: high latent heat values; higher thermal conductivity; not flammable; lower in cost in comparison to organic compounds; high water content means that they are inexpensive and readily available. However, their unsuitable characteristics have led to the investigation of organic PCMs for this purpose. These include: corrosiveness; instability; improper re-solidification; suffer from decomposition and super cooling affects their phase change properties [6,9-11]. As they require containment, they have been deemed unsuitable for impregnation into porous building materials [12].
Nucleating and thickening agents can be added to Inorganic Phase change materials to minimize super cooling and decomposition. Unlike conventional sensible thermal storage methods, PCMs provide much higher energy storage densities and the heat is stored and released at an almost constant temperature. PCMs can be used for both active and passive space heating and cooling systems [6].
Organic PCMs have a number of characteristics which render them useful for latent heat storage in certain building elements. They are more chemically stable than inorganic substances, they are non-corrosive, they have a high latent heat per unit weight, they are recyclable, they melt congruently and they exhibit little or no super cooling i.e. they do not need to be cooled below their freezing point to initiate crystallization.
Moreover, they have been found to be compatible and suitable for absorption into various building materials, as will be discussed in more detail later. Although the initial cost of organic PCMs is higher than that of the inorganic type, the installed cost is competitive. However, these organic materials do have their quota of unsuitable properties. The most significant of these characteristics is: low thermal conductivity, high changes in volume during phase change, they are flammable and they may generate harmful fumes on combustion. Other problems, which can arise in a minority of cases, are a reaction with the products of hydration in concrete, thermal oxidative ageing, odour and an appreciable volume change [6,12].
Appropriate selection and modification have now eliminated many of these undesirable characteristics. It has been found that the thermal oxidative ageing of PCMs concerned can be inhibited by the use of a proper antioxidant. Research is still underway to assess the flammability and fume generation of some of the more effective PCMs such that a fire rating may be established. Also, efforts are being made to extend the number of PCMs which are compatible with concrete [12].
PCMs have not always re-solidified properly, because some PCMs get separated and stratify when in their liquid state. When temperature dropped, they did not completely solidify, reducing their capacity to store latent heat. These problems are overcome by packaging PCM in containers and by adding thickening agents. To solve some of the problems inherent in inorganic PCMs, an interest has turned towards a new class of materials: low volatility, anhydrous organic substances such as paraffin’s, fatty acids and polyethylene glycol. Those materials were more costly than common salt hydrates and they have somewhat lower heat storage capacity per unit volume. It has now been realized that some of these materials have good physical and chemical stability, good thermal behaviour and adjustable transition zone [7,9,13].
In table 3 is presented the advantages and disadvantages of two main groups of PCMs.
Table 3. Advantages and disadvantages of PCMs
|
|
Organic (Paraffins) |
Inorganic (Salt Hydrates) |
|
Advantages |
-non-corrosive; -chemically and thermally stable; -no or little sub-cooling. |
-high melting enthalpy; -high density. |
|
Disadvantages |
-lower melting enthalpy; -lower density; -low thermal conductivity; -flammable. |
-sub-cooling; -corrosive; -phase separation; -phase segregation, lack of thermal stability; -cycling stability. |
Selection Criteria for PCMs
In order to select the best qualified PCM as a storage media some criteria’s are mentioned:
o Thermodynamic properties:
· Large enthalpy of transition with respect to the volume of the storage unit;
· High change of enthalpy near temperature of use;
· Phase change temperature fitted to application;
· The latent heat should be as high as possible to minimize the physical size of the heat storage;
· High latent heat of fusion per unit mass, so that a lesser amount of material stores a given amount of energy;
· A melting point in the desired operating temperature range;
· Fixed and clearly determined phase change temperature (freeze/melt point);
· Congruent melting point to avoid segregation;
· Lower change of volume during phase change;
· High density, so that a smaller container volume holds the material
· High thermal conductivity (both liquid and solid phases) would assist the charging and discharging of the energy storage high specific heat that provides additional sensible TES effect and also avoid sub cooling.
o Kinetic properties:
· Little or no undercooling during the freezing process;
· Sufficient crystallisation rates.
o Chemical properties:
· No chemical decomposition, so that the latent TES system life is assured;
· Non-corrosiveness to construction material;
· Long term chemical stability;
· Non-poisonous; Non-toxic;
· Non-explosive, non-dangerous;
· Non-flammable.
o Physical properties:
· Limited changes in density to avoid problems with the storage tank;
· High density with low density variation;
· Small units size;
· Low vapour pressure,
· Favourable phase equilibrium.
o Economic properties:
· Available in large quantities;
· Cheap in order to make the system economically feasible [6-10,13,14]
Micro and Macro Encapsulation Methods
PCMs have a relatively low thermal conductivity and in principle there are two ways of solving this problem. On one hand the distances for heat transfer by conduction in the PCM can be shortened. This can be done by encapsulating the material into relatively small capsules or by highly dispersed heat exchangers with low distances between fins or pipes. On the other hand the thermal conductivity can be enhanced by embedding structures of materials with high conductivity into the PCM. This is e.g. done by adding graphite powder into the PCM, which not only increases the thermal conductivity of the PCMs by a factor of 10-20, but also creates a kind of carrier structure that inhibits the segregation of salt hydrates and therefore improves their cycling stability [13].
There are two principal means of encapsulation: micro and macro encapsulation.
Micro-encapsulation enables to handle the PCMs independently of being solid or liquid. The microcapsules (figure 5) are tiny particles of solid, liquid or gas with diameters smaller than 1 mm and larger than 1μm (ussualy5–10μm in diameter), which surround the paraffinic PCM core material individually with a hard polymeric shell. The coated particles can then be incorporated in any matrix that is compatible with the encapsulating film. It follows that the film must be compatible with both the PCM and the matrix. Due to the small diameter the ratio of surface area to volume is very high and the low thermal conductivity is not a problem. If these microcapsules are dispersed in a fluid (mostly water), they form a pump able slurry, that can be used as an energy transport- and storage medium, as a so-called PCM slurry. Because of the small diameter of the microcapsules the slurry can be treated like a homogenous fluid. A microencapsulation of salt hydrates is not possible [11,13,15,16].
Considerable numbers of core and shell materials are now used to produce commercial microcapsules for different applications. This field of science and technology is growing very fast. Throughout the world research and development activities are dedicated to advancing microencapsulation technology. Dyes, drugs, fragrances and phase-change materials are very common materials for core and gum Arabic, amino plastics and ethyl cellulose are well used as shell materials [17].
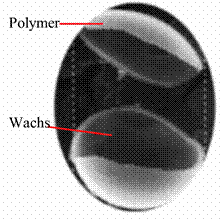
Figure 5. PCM Microcapsule
PCMs can be microencapsulated through a range of methods, both physical and chemical. Physical encapsulation methods include pan coating, air-suspension coating, centrifugal extrusion, vibration nozzle and spray drying. PCM is physically encapsulated via interfacial polymerization, in situ polymerization and matrix polymerization [18].
The second containment method is macro-encapsulation, which comprises the inclusion of PCM in some form of package such as tubes, pouches, spheres, panels or other receptacle. These containers can serve directly as heat exchangers or they can be incorporated in building products. The PCM must be encapsulated so that it does not adversely affect the function of the construction material. Previous experiments with large volume containment or macro-encapsulation failed due to the poor conductivity of the PCM. When it was time to regain the heat from the liquid phase, the PCM solidified around the edges and prevented effective heat transfer [19].
Both methods of PCM encapsulation in concrete (micro- and macro-encapsulation) may have some drawbacks. Plastic or metallic encapsulation of the PCM is expensive but safe, as the PCM is not in contact with the concrete. Microencapsulation by impregnating the PCM in the concrete is very effective, but it may affect the mechanical strength of the concrete [20].
Results and Discussion
The PCM can be used as natural heat and cold sources or manmade heat or cold sources. In any case, storage of heat or cold is necessary to match availability and demand with respect to time. There are three different ways to use PCMs for heating and cooling of buildings exist: PCMs in building walls; PCMs in building components other than walls i.e. in ceilings and floors; and PCMs in separate heat or cold stores [7].
The first two are passive systems, where the heat or cold stored is automatically released when indoor or outdoor temperatures rise or fall beyond the melting point. The third one is active system, where the stored heat or cold is contained thermally separated from the building by insulation. Therefore, the heat or cold is used only on demand and not automatically. In building applications, only PCMs that have a phase transition close to human comfort temperature (20–28ºC) can be used. Some Commercial PCMs have been also developed for building application [7,8,19]. Commercial PCMs suitable for building applications are presented in table 4.
Table 4. Phase Change Temperature and Heat of Fusion of Typical Commercial PCMs
|
PCM Name |
Type of Product |
Melting Temperature [ºC] |
Heat of Fusion [kJ/kg] |
|
Astorstat HA17 |
Paraffins and Waxes |
21.7-22.8 |
- |
|
Astorstat HA18 |
Paraffins and Waxes |
27.2-28.3 |
- |
|
RT26 |
Paraffin |
24-26 |
232 |
|
RT27 |
Paraffin |
28 |
206 |
|
Climsel C23 |
Salt Hydrate |
23 |
148 |
|
Climsel C24 |
Salt Hydrate |
24 |
108 |
|
STL27 |
Salt Hydrate |
27 |
207 |
|
S27 |
Salt Hydrate |
29 |
188 |
|
- |
Mixture of Two Salt Hydrate |
22-25 |
- |
|
E23 |
Plus ICE (mixture of Non-Toxic Euretic Solution |
23 |
155 |
Thermal energy storage in the walls, ceiling and floor of the buildings may be enhanced by encapsulating or embedding suitable PCMs within these surfaces. They can either capture solar energy directly or thermal energy through natural convection. Increasing the thermal storage capacity of building can increase human comfort by decreasing the frequency of internal air temperature swings so that indoor air temperature is closer to the desired temperature for a longer period of time [8]. Some application areas for PCM in buildings are illustrated in Figure 6: No. 1: Latent heat store for space heating. No. 2: Plaster and compound systems with high heat storage capacity. No. 3: Transparent insulation and day lighting schemes. No. 4: Shading PCM compounding system. No. 5: PCM in gypsum products and paints. No. 6: PCM to buffer temperature variations in solar-air systems [21].
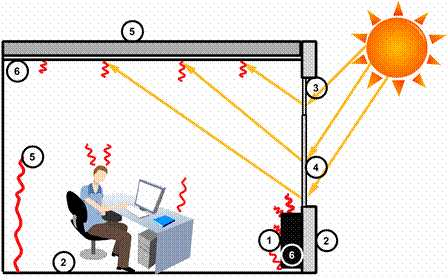
Figure 6. Application areas for PCM in buildings
Among all the PCM applications for high performance buildings, the PCM integrated wall is most commonly studied and concerned due to its relatively more effective heat exchange area and more convenient implementation. Generally speaking, there are two ways to integrate phase change materials with building walls: “immersion” and “attachment”. The solution of “immersion” is to integrate the phase change materials with the construction material of the building envelope, such as concrete, bricks and plaster. There are normally three ways to immerse PCM with the building construction material: direct immersion, macro-encapsulated PCM and micro-encapsulated PCM [22]. Other solution is to attach one or several PCM integrated wallboard layers to the wall. In this case, the PCM does not constitute the material of wall, but is integrated with the attached layers beyond the wall. As PCM is only integrated with the wallboard instead of the main wall, it can be considered as part of the indoor decoration work after the construction of building envelopes. The separate PCM layer, such as PCM integrated gypsum board and PCM integrated composite panel, allows a separate mass production of certain wallboards by typical companies; thus, increase the efficiency and reduce the overall cost [23].
Ceiling boards are the important part of the roof, which are utilized for the heating and cooling in buildings. PCM assisted ceiling system is more utilized building application due to its easier installation and implementation with the envelope. Generally speaking, there are currently three types of PCM assisted ceiling systems: PCM slurry assisted ceiling system; PCM integrated ceiling system, and separate PCM-storage-unit assisted ceiling system/air-conditioning system.
Floor is also the important part of a building and heating and cooling of buildings were tried using it. Electrical under-floor heating system is one of the most commonly used methods to provide heat. In many countries, the electricity tariffs are different between peak hours (usually during daytime with high-tariff) and off peak hours (usually during night time with low-tariff).
A major development in this area is to develop a PCM which will maintain good heat storage during the day and heat loss to the environment during night time [7]. The use of a complete solid-liquid-vapour phase change cycle will further increase the storage density. Such systems are technically feasible, but quite a bit more complicated than the simple (and passive) solid-liquid-solid cycle [21].
PCM Solar Wall
A PCM wall is capable of capturing a large proportion of the solar radiation incident on the walls or roof of a building. Because of the high thermal mass of PCM walls, they are capable of minimizing the effect of large fluctuations in the ambient temperature on the inside temperature of the building. They can be very effective in shifting the cooling load to off-peak electricity period [19, 23].
The wall consists of six main components: glass, transparent insulation material, polycarbonate, ventilated air channel, insulation and plaster (figure 7). Short wave radiation passes through glass with transparent insulation material, which prevents convective and thermal radiation heat transfer. Phase change material in a transparent plastic casing made of polycarbonate, absorbs and stores energy mostly as latent heat. The air for the house ventilation is heated in the air channel and it is led into the room. Insulation and plaster are standard elements.
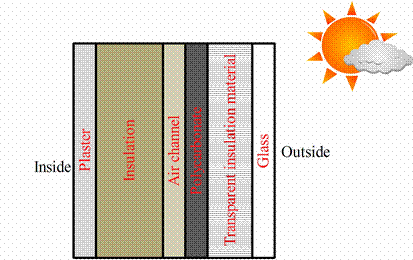
Figure 7. Elements of PCM solar wall
PCM Integrated in Wood – Light Weight – Concrete
Wood –lightweight- concrete is a mixture of cement, wood chips or saw dust, which should not exceed 15 % by weight, water and additives. This mixture can be applied for building interior and outer wall construction. The incorporation of PCM has two additional reasons: to increase the thermal storage capacity and to get lighter and thinner wall elements with improved thermal performance [19]. It was shown that PCMs can be combined with wood-lightweight-concrete and that the mechanical properties do not seem to change significantly. The authors reported the following advantages:
· Thermal conductivity: λ between 0.15 and 0.75 W/m K;
· Noise insulation;
· Mechanical properties: density between 600 and 1700 kg/m3;
· Heat capacity cp within 0.39 to 0.48 kJ/kg K at ρ =1300 kg/m3;
· Density about 60-70% of the value of pure concrete (0.67 kJ/kg K at ρ = 2400 kg /m3) [24].
PCM Filled Glass Windows
Most of the studies and applications have focused on the “opaque” part of building envelopes, such as walls, ceilings, and floors. However, we should notice one fact: generally speaking, “transparent” part of the building envelopes, i.e. window, has much lower thermal resistance than other parts of the envelopes.
Ismail et.al [26] proposed a different concept for thermally effective windows using a PCM moving curtain, as shown in figure 8. The window is double sheeted with a gap between the sheets and an air vent at the top corner. The sides and bottom are sealed with the exception of two holes at the bottom, which are connected by plastic tube to a pump and the PCM tank. The pump is connected in turn to the tank containing the PCM, which is in liquid phase. The pump operation is controlled by a temperature sensor. When the temperature difference reaches a pre-set value the pump is operated and the liquid PCM is pumped out of the tank to fill the gap between the glass panes. Because of the lower temperature at the outer surface, the PCM starts to freeze, forming a solid layer that increases in thickness with time and hence prevents the temperature of the internal ambient from decreasing. This process continues until the PCM changes to solid. A well designed window system will ensure that the external temperature will start to increase before the complete solidification of the enclosed PCM [19,26].
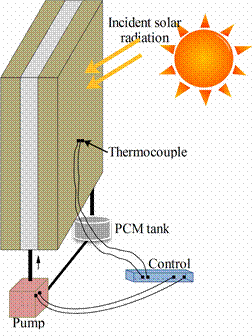
Figure 8. PCM filled glass windows
This concept of the PCM filled window system is viable and thermally effective. The PCM filling leads to filtering out the thermal radiation and reduces the heat gain or losses because most of the energy transferred is absorbed during the phase change of the PCM. The double glass window filled with PCM is more thermally effective than the same window filled with air.
PCM Assisted Sun-Shading
The PCM utilized in PCM assisted sun-shading system is hydrated salt CaCl2·6H2O. This system is very suitable to be utilized under the hot summer climate, especially for those areas with signifiant daytime and nighttime temperature fluctuations [27]. In figure 9 is presented conventional and PCM sun-shading system.
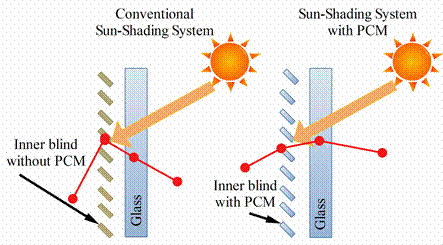
Figure 9. Schematic of the sun-shading systems with and without PCM
During the daytime with high temperatures (compared to the thermal comfort value), the face of the inner blind integrated with PCM is rotated to be exposed to the solar radiation so that excess solar energy is stored in PCM, attenuating the temperature fluctuations inside the room.
During the night time with relatively low temperatures (compared to the thermal comfort value), the face of the inner blind integrated with PCM is rotated to be exposed to the room air so that the stored energy is released back to the room, avoiding over-reduction of the room temperature below the thermal comfort value.
PCM Assisted Under-Floor Electric Heating System
In order to investigate the thermal performance of the under-floor electric heating system with the shape-stabilized PCM plates, an experimental house with this system was set up in Tsinghua University, Beijing, China. The experimental house was equipped with the under-floor electric heating system including shape stabilized PCM plates. It had a double-glazed window facing south, covered by black curtain. The roof and walls were made of polystyrene wrapped by metal board. The under-floor heating system included polystyrene insulation, electric heaters, PCM, some wooden supporters, air layer and wood floor [19].
Under-floor electric heating system with shape-stabilized PCM plates is presented in figure 10. Different from conventional PCM, shape-stabilized PCM can keep the shape unchanged during phase change process. Therefore, the PCM leakage danger can be avoided. This system can charge heat by using cheap nighttime electricity and discharge the heat stored at daytime [25].
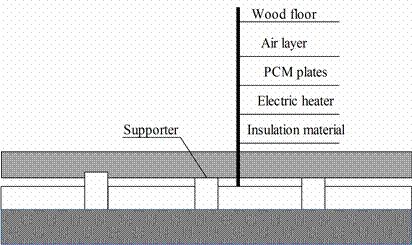
Figure 10. Schematic of electric floor heating system
PCM Integrated Roof
A roof-integrated solar air heating/storage system uses existing corrugated iron roof sheets as a solar collector for heating air. A PCM thermal storage unit is used to store heat during the day so that heat can be supplied at night or when there is no sunshine. The system operates in three modes. During times of sunshine and when heating is required, air is passed through the collector and subsequently into the home. When heating is not required air is pumped into the thermal storage facility, melting the PCM, charging it for future use. When sunshine is not available, room air is passed through the storage facility, heated and then forced into the house. When the storage facility is frozen, an auxiliary gas heater is used to heat the home. Adequate amounts of fresh air are introduced when the solar heating system is delivering heat into the home as shown figure 11 [23].
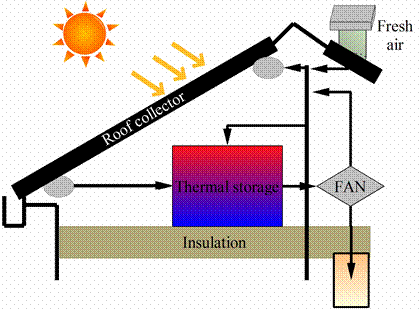
Figure 11. Schematic of the solar heating system
PCM Assisted Ceiling
PCM asisted ceiling was investigated at the University of Nottingham (2002). This is a replacement of a full air conditioning system by the new system that is a nighttime cooling system, which is also easy to retrofit. The proposed module (figure 12) it is ceiling-mounted with a fan to throw air over the exposed ends of heat pipes. The other end of the heat pipes is in a PCM storage module. During the day, the warm air generated in the room is cooled by the PCM i.e. heat is transferred to the PCM. During the night, the fan is reversed and the shutters are opened such that cool air from the outside passes over the heat pipes and extracts heat from the PCM. The cycle is then repeated next day [19].
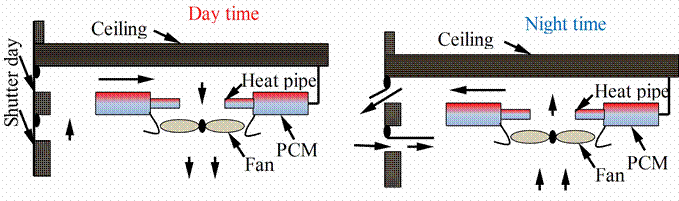
Figure 12. System design as proposed by the University of Nottingham
PCM Integrated In Combined Heating and Cooling System
The Sustainable Energy Centre at University of South Australia (2000) started work with PCMs in the mid 1990’s with the development of a storage unit that can be used for both space heating and cooling. The night time charging and day time utilization process during both heating and cooling seasons for a storage system comprising of two different PCMs integrated into a reverse cycle refrigerated heat pump system utilizing off peak power. As the air is forced through the system it undergoes a two-stage heating or cooling process. It first goes through one PCM and then the second as shown in figure 13 [23].
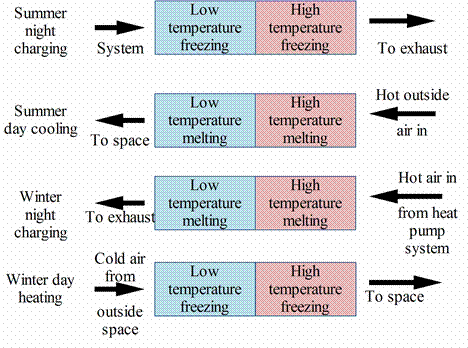
Figure 13. Night-time charging and day-time utilization process during both heating and cooling seasons
The melting / freezing point of the first material are below comfort temperature, while the second material has a melting/freezing point above comfort temperature. During the winter, the airflow is adjusted so that the system stores heat at night (by both materials melting) and releases heat at a temperature above comfort conditions (by freezing) at daytime. During summer, the airflow direction is reversed and the system stores cold energy at night and it releases the cool air below comfort temperature at daytime [23].
Conclusions
The incorporation of PCMs into building elements takes the advantage of latent TES for additional energy savings. The development of energy-storing building is a solution to the on-going quest for energy conservation, and also to improving the indoor environment in which people work and live. In terms of thermal comfort, it is envisaged that the indoor environment of a building which uses PCM construction materials will have significantly lower mean radiant temperatures and more thermal stability, having less likelihood of overheating and fewer temperature fluctuations.
Thermal improvements in a building due to the inclusion of PCMs depend on the type of PCM, the melting temperature, the percentage of PCM mixed with conventional material, the climate, design and orientation of the construction of the building. The optimization of these parameters is fundamental to demonstrate the possibilities of success of the PCMs in building materials.
References
1. Abedin A.H., Marc A. Rosen, A Critical Review of Thermochemical Energy Storage Systems, The Open Renewable Energy Journal, 2011, 4, p. 42-46.
2. Pavlov G., Olesen B.W., Building thermal energy storage- concepts and applications, Available at: http://orbit.dtu.dk/fedora/objects/orbit:72784/datastreams/file_6383088/ content, (accessed 10/05/2012).
3. Dincer I., Rosen M., Thermal energy storage. Systems and applications, Ed. Wiley, second edition, 2011.
4. Demirbas M.F., Thermal Energy Storage and Phase Change Materials: An Overview, Energy Sources, Part B, 2006, 1, p. 85–95.
5. Mondal S., Phase change materials for smart textiles- An overview, Applied Thermal Engineering, Available at: http://www.ecaa.ntu.edu.tw/weifang/pcm/Phase%20change %20materials%20for%20smart%20textiles%20%E2%80%93%20An%20overview%20(2008).pdf, 2008, 28, p. 1536-1550, (accessed 10/05/2012).
6. Karthikeyan S.,Muthu Saravanan S., Prashanth R., Energy conservation through phase change material based thermal energy storage system-a project report, Anna University Chennai, 2006.
7. Ravikumar M., Srinivasan Dr.Pss., Phase change material as a thermal energy storage material for cooling of building, Journal of Theoretical and Applied Information Technology, 2005-2008, 4(6), p. 503-512, Available at: http://www.jatit.org/volumes/research-papers/Vol4No6/6Vol4No6.pdf, (accessed 10/05/2012).
8. Ravikumar M., Srinivasan Dr.Pss, Natural cooling of building using phase change material, International Journal of Engineering and Technology, 2008, 5(1), p. 1-10, Available at: http://ijet.feiic.org/journals/J-2008-V1001.pdf, (accessed 10/05/2012).
9. Zalba B., Marın J.M., Cabeza L.F., Mehling H., Review on thermal energy storage with phase change: materials, heat transfer analysis and applications, Applied Thermal Engineering 23, 2003, p.251–283.
10. Patil N.D., Design and Analysis of Phase Change Material based thermal energy storage for active building cooling: a Review, International Journal of Engineering Science and Technology (IJEST), 2012, 4(6), p.2502-2509, Available at: http://www.ijest.info/docs/IJEST12-04-06-027.pdf, (accessed 10/06/2012).
11. Khudhair A.M., Farid M.M., A review on energy conservation in building applications with thermal storage by latent heat using phase change materials, Energy Conversion and Management 2004, 45, p. 263-275.
12. Ruth Kelly, Latent heat storage in building materials, Available at: http://www.cibse.org/pdfs/Latent%20heat%20storage.pdf, (accessed 10/05/2012).
13. Heinz A., Streicher W., Application of phase change materials and PCM-slurries for thermal energy storage, Available at: http://talon.stockton.edu/eyos/energy_studies /content/docs/FINAL_PAPERS/8B-4.pdf, (accessed 10/05/2012).
14. Reddy R.M., Nallusamy N., Prasad B.A., Reddy K. H, Thermal Energy Storage System using Phase Change Materials-Constant Heat Source, Available at: http://www.doiserbia.nb.rs/img/doi/0354-9836/2010%20OnLine-First/0354-98361000078R.pdf, (accessed 10/05/2012).
15. Ekkehard J., Burger J., Micro-encapsulated phase change material– manufacture, properties and utilization, Available at: http://www.inda.org/events/training/reading/ SuggestedReadings/Microencapsulated%20Phase%20Change%20.pdf, (accessed 10/05/2012).
16. Kośny J., Yarbrough D.W., Development and testing of ignition resistant microencapsulated phase change material, Available at: https://talon.stockton.edu/ eyos/energy_studies/content/docs/effstock09/Session_4_2_PCM_in_Buildings/33.pdf, (accessed 10/05/2012).
17. Fallahi E., Barmar M., Haghighat Kish M., Preparation Of Phase-Change Material Microcapsules With Paraffin Or Camel Fat Cores: Application To Fabrics, Iranian Polimer Journal, 2010, 19(4), p. 277-286, Available at: http://journal.ippi.ac.ir, (accessed 10/05/2012).
18. Whiffen T.R., Riffat S.B., A review of PCM technology for thermal energy storage in the built environment: Part I, International Journal of Low Carbon technologies Advance Access, 2012, 0, p.1-12, Available at: http://ijlct.oxfordjournals.org/content/early/ 2012/05/30/ijlct.cts021.full.pdf+html, (accessed 30/05/2012).
19. Velraj R., Pasupathy A., Phase change material based thermal storage for energy conservation in building architecture, Available at: http://celsius.co.kr/phase_change_materials/download/energy/PCM_based_thermal_storage_for_energy_conservation_in_building_architecture.pdf, (accessed 10/05/2012).
20. Castellon C., Medrano M., Roca J., Nogues M., Castell A., Cabeza L.F., Use of Microencapsulated Phase Change Materials in Building Applications, Available at: http://www.ornl.gov/sci/buildings/2012/Session%20PDFs/35_New.pdf, 2007 (accessed 10/05/2012).
21. Nielsen K., Thermal Energy Storage A State-of-the-Art- A report within the research program Smart Energy-Efficient Buildings at NTNU and SINTEF 2002-2006, Available at: http://www.scribd.com/doc/23310231/Storage-State-of-the-art, 2003, (accessed 10/05/2012).
22. Sharma A., Tyagi V.V., Chen C.R., Buddhi D., Review on thermal energy storage with phase change materials and applications, Renewable and Sustainable Energy Reviewa, 2009, 13, p. 318-345.
23. Sunliang C., State of the art thermal energy storage solutions for high performance buildings, Master`s Thesis, university of Jyvaskyla, Department of physics, Master`s Degree Programme in Renewable Energy, 2010.
24. Mehling H., Krippner R., Hauer A., Research project on PCM in wood-lightweight-concrete, IEA, ECES IA Annex 17, Advanced Thermal Energy Storage through Phase Change Materials and Chemical Reactions - Feasibility Studies and Demonstration Projects. 2nd Workshop, Ljubljana, 3-5 April, Slovenia, 2002.
25. Lin K., Zhang Y., Xu X., Di H., Yang R., Qin P., Experimental study of under-floor electric heating system with shape-stabilized PCM plates, Energy and Buildings, 2005, 37, p. 215-220.
26. Ismail K.A.R., Henriquez J. R., Thermally effective windows with moving phase change material curtains, Applied Thermal Engineering, 2001, 21, p. 1909-1923.
27. Mehling H., Stategic project “Innovative PCM-Technology” Results and future perspectives, 8th Expert Meeting and Workshop, Kizkalesi, Turkey, 2005, Available at: http://www.fskab.com/Annex17/Workshops/EM8%20Kizkalesi/Presentations/Innovative%20PCM-Technology.pdf, (accessed 10/05/2012).