Suitability of Structural Aluminium Profiles as Sacrificial Anode for Carbon Steel
Iyiola O. OTUNNIYI* and Daniel T. OLORUN TOBA
Metallurgical
and Materials Engineering,
E-mail: ioogunniyi@futa.edu.ng
* Corresponding author: Phone: +2348132970816
Abstract
The life of low carbon steels in many small scale structures can be remarkably prolonged by complementary sacrificial anode cathodic protection (SACP) but for constraints in availability of specialty anodes. Suitability of commonly available structural aluminium profiles as sacrificial anodes for low carbon steel has been investigated. Three different profiles were studied. Bare and coated steel samples were found to migrate clearly too cathodic potential regimes when coupled with the press-finish BS1470:6000 AlMgSi series alloy in a chloride medium. No weight loss was observed for the coated steel sample, while the aluminium profile showed dissolution. This alloy, commonly available in press-finish profiles for structural purposes, is therefore recommendable as sacrificial anodes for complementary SACP of low carbon steel structures under atmospheric or aqueous exposures.
Keywords
Sacrificial anode; Low carbon steel; Anode materials; Aluminium alloy.
Introduction
Carbon steel is a remarkably cheap material, with good tensile strength (350 - 850 N/mm2 in normalised plain carbon steels), and easy to weld using gas, arc or resistance welding [1]. In applications, these factors often weigh in, making carbon steels about the most used alloys, with world crude steel production reaching 1240 mmt in 2009 [2-3]. The recurring challenge with the use of carbon steels is the poor corrosion resistance. In open atmosphere and in aqueous media, overall uniform corrosion, crevice corrosion, pitting, and other modes of corrosion readily sets in [2, 4]. Various corrosion prevention methods, ranging from physical isolation such as coating and painting to electrochemical techniques, are employed to prolong the service life of carbon steel members [2, 4].
For structures relying on painting and coatings only, complete protection cannot be guaranteed because, more often than not, there are holidays on coated and painted surfaces which serve as small anode sites relative to the rest of the large covered area that will be relatively cathodic. Gradually, paint and coating debonding and peeling also do occur due to surface imperfection (surface energy, dirt, relief), mechanical damage and with change climatic conditions. These are requisite conditions for crevices, pitting, differential earation, and filiform corrosion damages that with common with coated and or painted carbon steel surfaces [1,5,6]. Complementary electrochemical protection such as cathodic protection is normally recommended and employed with painted or coated carbon steel [2]. However, in practice, such complementary protection is often employed to prevent only high integrity structures oil platforms, ships and such. It is generally rare to find cathodic protection on commonplace private small scale structures that is supposed to have been so protected. Such structures rely only on physical protection. Active corrosion damage therefore continues everyday at these small scale levels, which cumulatively is a very large amount of corrosion damage on the global scale.
Considering the cathodic protection option, the sacrificial anode cathodic protection (SACP) is relatively simple and easy to install compared to impressed currents. SACP electrochemically protects the integrity of the whole structure as long as the sacrificial anode remains coupled and is not yet used up. There is no need of a power source. SACP should be in widespread usage on small scale structures, but cost and availability of the specialty anode alloys is a major constraint.
In choice of anode, it is basically required that the anode is less noble of lower electrode potential than the alloy it is to protect. The current capacity and the anode life are other factors in comparative choice making. Pure aluminium, zinc and magnesium at standard electrode potentials of −1.66, −0.763 and −2.37 V (SHE) respectively, are all candidate anodes for carbon steels with standard electrode potential typically −0.44 V (SHE) [7]. Zinc has been the orthodox anode material for steel in sea water [8]. Magnesium although gives the highest electrode potential difference relative to mild steel, but the current efficiency of magnesium anode is about 50 60 %, compared with zinc or aluminium alloys which have efficiencies greater than 90 % [8]. With trivalent cation, aluminium releases three faradays per mole on oxidation, implying a higher anode current capacity (protection ampere-hours supplied per kilogram anode). However, the characteristic tenacious surface oxide of pure aluminium is protective and can limit or make current output unpredictable [8].
Specialty aluminium alloys were later developed to prevent formation of the continuous adherent oxide film, allow continuous galvanic activity and serve as good anodes. Such alloys often contain zinc with mercury or indium. Table 1 shows composition of some anode alloys of aluminium, zinc and magnesium. Comparing these alloys based on current capacity, Galvalum I gives 264 Ahkg-1. The US Navy conventional and low voltage aluminum alloy sacrificial anodes are qualified for least current capacity of 2535 and 1656 Ahkg-1 respectively [9]. The zinc alloy C-Sentry and magnesium alloy Galvomag (Table 1) have current capacity of 780 and 1232 Ahkg-1[8]. The advantage of the aluminium anodes over zinc and magnesium is obvious. On general basis of anode current capacity and anode consumption rate, aluminium alloys have been preferred for steel SACP [10,11].
Table 1. Elemental composition of sacrificial anode alloys
|
Aluminum[8] (Galvalum I) |
Aluminum[9] (Conventional) |
Aluminum[9] (Low Voltage) |
Zinc[8] (C-Sentry) |
Magnesium[8] (Galvomag) |
|
Al: Bal. |
Al: Bal. |
Al: Bal. |
Al: 0.4- 0.6 |
Al: <0.01 |
|
Cu: <0.006 |
Cu: ≤0.004 |
Cu: ≤0.005 |
Cd: 0.075 0.125 |
Cu: 0.02 |
|
Fe: <0.1 |
Fe: ≤0.090 |
Fe: ≤0.080 |
Cu: <0.005 |
Fe: <0.03 |
|
Hg: 0.02-0.05 |
Hg: ≤0.001 |
Hg: <0.005 |
Fe: <0.0014 |
Mg: Bal. |
|
Si: 0.11-0.21 |
In: 0.014-0.020 |
In: <0.005 |
Pb: <0.15 |
Mn: 0.5 -1.3 |
|
Zn: 0.3-0.5 |
Si: 0.08 0.2 |
Mg: <0.01 |
Si: <0.125 |
Ni: 0.001 |
|
Others: Each <0.02 |
Sn: ≤0.001 |
Mn: <0.01 |
Zn: Bal. |
Pb: <0.01 |
|
Zn: 4.0- 6.5 |
Ni: <0.005 |
Sn: <0.01 |
||
|
Si: <0.1 |
Zn: <0.01 |
|||
|
Sn: ≤0.001 |
||||
|
Zn: <0.15 |
||||
|
Ga: 0.092-0.110 |
However, when these speciality alloys are outreach, it is necessary to be able to recommend a makeup of some sort. Ready availability of anodes will be very helpful in making application of cathodic protection too many domestic and commonplace structures feasible in many parts of the globe. This work is therefore to assess the suitability of some structural aluminium profiles as cheap, readily available anodes for carbon steel. Low carbon steel and chloride medium were considered as typical of steels and possible corrosive environments. Low carbon steel is often encountered and more susceptible to corrosion than high carbon steels, while the chloride environment is a relatively more corrosive environment compared with normal atmospheric wet condition. The BS1470:6000 series AlMgSi aluminium alloy have 235 % elongation at brake, and 40 - 455 tensile strength [1,12]. With this mechanical property, this alloy is used extensively in extruded profiles. It is therefore readily available as different structural profiles with different surface finish for various target applications.
The aim of this research paper was to investigate the suitability of different profiles of the structural aluminium alloy as sacrificial anodes for low carbon steel.
Material and Method
Low carbon steel, a very common engineering material was also obtained. The chemical compositions of the low carbon steel and aluminium profiles are shown in Table 2. Press-finish, anodized and powder coated extruded aluminium profiles were obtained. The compositions of the profiles obtained closely approximate that of the BS1475:6063 aluminium alloy [1]. The presence of zinc in the aluminium alloy is expected to be advantageous for the galvanic behaviour of this alloy com-pared to a strictly 6063 alloy with no zinc content [9-11].
Based on the observations from this initial study as discussed in the results below, the press-finish aluminium profile was considered as more promising for use as an anode. Samples of the press-finish aluminum profile were then coupled with samples of the bare and nickel coated low carbon steel in 0.5 M chloride solution. The test anode and the samples were connected before immersion in the solution.
The electrode potential of the steel samples and the aluminium profiles in the aqueous environment were separately monitored everyday for 15 days, while weight loss data of the samples were taken at three days interval over the period. For the weight loss, the samples were removed from their various chloride environments, rinsed in distilled water and dried with cotton wool.
Table 2. Chemical composition (wt %) of low carbon steel and aluminum
|
Element |
Weight % |
|
|
Structural Aluminum |
Low Carbon Steel |
|
|
Fe |
0.45 |
98.72 |
|
C |
- |
0.181 |
|
Si |
0.45 |
0.056 |
|
Mn |
0.2 |
0.474 |
|
S |
- |
0.047 |
|
P |
- |
0.039 |
|
Cu |
0.2 |
0.198 |
|
Al |
98.83 |
0.124 |
|
Ni |
- |
0.078 |
|
Hg |
|
|
|
Cr |
0.2 |
0.038 |
|
Ti |
0.2 |
0.01 |
|
Mg |
0.05 |
- |
|
Zn |
0.2 |
- |
The dried samples were weighed with a 0.0001 g precision digital weighing balance (Scout Pro SPU402 model). The weight losses were recorded and the cumulative weight loss was calculated. The corrosion rate was determined as [13]:
|
R = W/A(T/365) |
(1) |
where R = the corrosion rate (mg/mm2/year), W= the weight difference, A= the area of the specimens (mm2), and T = the exposure time in days.
Results and Discussion
Various Profiles as Sacrificial Anode for Low Carbon Steel
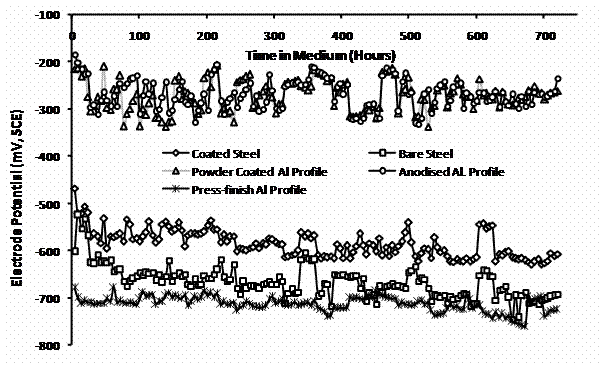
Figures 1 show the electrode potentials of the various aluminium profiles over time in 0.5 M NaCl solution, all potentials being relative to the silicon calomel electrode (SCE). From beginning of immersion into the medium, with electrode potential about −200 mV, the anodised and the powder coated aluminium profile, both show to be of more noble potentials than the bare and the electroplated steel samples that gave electrode potentials of −600 and −460 mV, respectively. Over time, the anodised and the powder coated aluminium profiles dropped to fluctuate in between −200 and −340 mV, yet the values remain clearly well above those for the steel samples all over the 720 hours for the observations. Over the time, bare steel electrode potential dropped to fluctuate at values below −600 mV. The press-finish aluminium profile on the other hand, from the beginning of immersion, gave about −675 mV. The value dropped to about −700 mV and fluctuated narrowly around this value over the time range.
|
|
|
Figure 1. Electrode potential over time in 0.5M NaCl solution for various aluminium profiles, and nickel-plated and bare steel samples |
With this electrode potential values and responses, only the press-finish aluminium profile can be considered as a potential sacrificial anode material for cathodic protection of the steel samples such medium. The anodised and the powder coated aluminium profiles remain clearly nobler than the steel samples all through. Although all the aluminium profiles are from the structural alloy compositions (Table 2), anodising and powder coating are surface treatments already in place to make the profiles resistant to atmospheric corrosion [1,14,15]. In the anodised profile, the natural oxide layer of aluminium has been thickened by anodising treatment to ensure protection of the pure metal beneath [1,8]. The powder coated profile also has a thin coating formed from organic powder sprayed and cured in place on preheated metal surface [16]. This treatment provides effective covering for the reactive metal surface. The observations thus show the effect of these treatments on the electrode potential on the otherwise less noble alloy. The anodised and the powder coated profiles therefore cannot serve as an anode for the steel.
SACP of Steel Samples with Press-Finish Aluminium
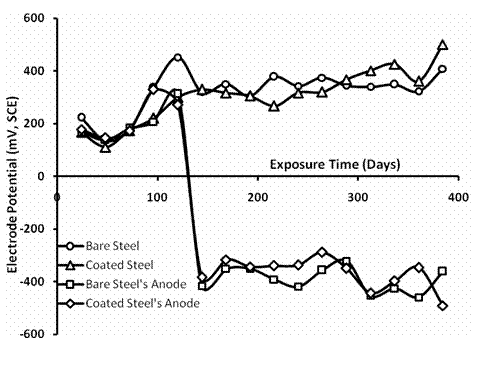
Figure 2 shows the electrode potential changes over time when the bare and the coated steel samples were separately coupled with press-finish aluminium as sacrificial anode for cathodic protection in a chloride environment. The response shows that the press-finish aluminium clearly became anodic to the steel sample and gave sacrificial protection. The aluminium and the steel samples on immersion, all being in electrical contact first gave similar response, hovering around very close electrode potential values. After about five days, the electrode potentials of the aluminium profiles connected to the bare and the electroplated steel both nosedived sharply in a few hours to the region of −400 mV. It continued to fluctuate about these values for the rest of the measurements. The steel samples on the other hand show cumulative slight increase in electrode potentials. This is indicative of more cathodic state for the steels in the medium over time relative to the aluminium profiles.
|
|
|
Figure 2. Electrode potentials over time in 0.5 M NaCl for bare and nickel plated carbon steel samples, and press-finish aluminium as sacrificial anodes |
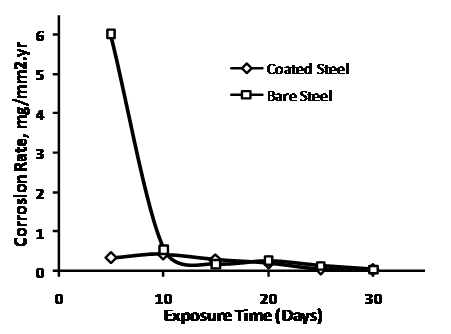
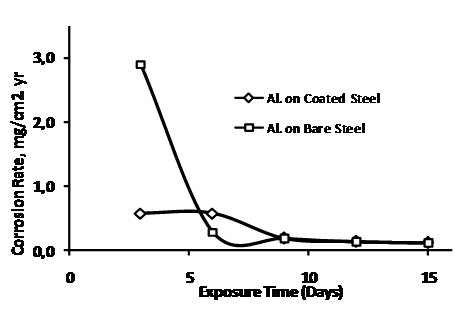
Comparing the mass changes (weight loss) in the sample, Figure 3 shows the corrosion rate in the bare and the coated steels. Expectedly, the coated steel show much lower corrosion rate. However, the fact that the nickel plated samples still shows measureable corrosion shows that corrosion prevention methods such as coating, relying on physical isolation of the surface from its environment, do not offer perfect protection. Tiny holidays will often be present, producing a small anode to cathode surface and resulting galvanic activities. The coating becomes cathodic to the substrates and makes the corrosion even faster than if the surface had been bare. After the steels were coupled cathodically with the pressed finished aluminium anodes, no measurable corrosion rate was observed. Rather measurable mass changes (corrosion rate) can be recorded on the press finish aluminium alloy cathodically coupled with the steel samples (Figure 4). This clearly indicates the sacrificial protection offered by the aluminium profile. The pressed-finish aluminium alloy, though not a specialty alloy for sacrificial anodes, therefore demonstrated a level of suitability to serve as anode for low carbon steel. The presence of zinc in the composition can be considered as enhancing galvanic activity in this profile, zinc being a major alloying constituent in Galvalum I (Table 1) and an activator in aluminium anodes [7, 17, 18].
|
|
|
Figure 3. Corrosion rate for bare and nickel plated low carbon steel samples over time in 0.5 M NaCl |
|
|
|
|
|
Figure 4. Corrosion rate for press-finished aluminium anode catholically coupled with bare and nickel plated low carbon steel samples over time in 0.5M NaCl |
Application of the pressed-finished profile as sacrificial anode for low carbon steel is therefore recommendable for many domestic, small scale and medium scale steel structures in many places where such structures rely only on painting or coating. There are many factors that can cause a painting or a coating to fail [19]. After coating failure, differential aeration typically sets in, with corrosion at the paint-metal interfaces [2, 8]. A complementary SACP as observed herein will prevent such situation and prolong the life of paints and the whole structure. It is easy to imagine the high volume of domestic corrosion failure in very many urban and sub-urban private structures, which is cumulatively as enormous as that occurring on few large industrial set structures. While the heavy industrial structures can afford specialty anodes, it is advisable for small to medium scales to use readily available alloys and improve on the level of protections on their structures.
Conclusions
Many steel structures in private, small and medium scales installations rely only on paintings and coating for corrosion prevention, which ultimately do fail. Industrial scale structures use complementary protections such as SACP, with specialty alloys as anodes. Towards more domestic application of SACP, suitability of readily available aluminium alloys, available as structural profiles, as sacrificial anodes has been investigated. The press-finish aluminium profile of the BS1470:6000 AlMgSi series has been found suitable. Bare and coated steel samples clearly migrated to cathodic potential regimes when coupled with the press-finish aluminium in a chloride medium. No weight loss was observed for the coated steel sample, while the aluminium showed dissolution. This press-finish aluminium profile is readily available and is therefore recommendable as sacrificial anodes for complementary SACP of many low carbon steel structures in private applications relying only on painting and coating under atmospheric or aqueous exposures.
References
1.
Higgins R.A., Engineering
Metallurgy: Applied Physical Metallurg,
2. Roberge P.R., Handbook of Corrosion Engineering, USA, Mc GrawHill, vol.1, p.736-1011, 1999.
3. DiFrancesco C.A., Kelly T.D., Bleiwas D.I., Fenton M.D., Iron and Steel Statistics, US Geological survey, 2010.
4. Bryson J.H., Corrosion of Carbon Steels, in Corrosion, ASM Handbook, 9 ed., Vol. 13, ASM International, p. 1220, 1987.
5. Kelly R.G., Crevice Corrosion, Corrosion: Fundamentals, Testing, and Protection, Vol. 13A, ASM Handbook, ASM International, p 242247, 2003.
6. Hahin C., Buchheit R. G., Filiform Corrosion, in Corrosion: Fundamentals, Testing, and Protection, ASM Handbook Vol. 13A, ASM International, p 248256, 2003.
7. Protopopoff E., Marcus P., Electrode Potentials, in Corrosion: Fundamentals, Testing, and Protection, Vol 13A, ASM Handbook, ASM International, p.812, 2003.
8. Tretheway K.R., Chamberlain J., Corrosion for Science and Engineering, 2nd Edition, Essex, England, Group Longman Limited, p.384, 1998.
9.
10. Heidersbach R.H., Cathodic Protection, in Corrosion: Fundamentals, Testing, and Protection, Vol. 13A, ASM Handbook, ASM International, p. 855870, 2003.
11.
Wigg M., Fleury P., Whats the
Truth about Aluminum Sacrificial Anodes?, Available at: www.liboatingworld.com/archive/2006/01/LIBW/LIBW_65.pdf
(accessed
12.
MatWeb: Overview of
Materials for 6000 series Aluminium Alloys, Available at: www.matweb.com (accessed
13.
Fontana M.G., Corrosion
Engineering, 2nd Edition,
14. Scamans G.M., Bribilis N., Buchheit R.G., Corrosion of Aluminium and its Alloys, in Shreirs Corrosion, vol. 3, p. 19742010, 2010.
15. Schuman T.P., Protective coatings for Aluminium alloys, Handbook of Environmental Degradation of Materials, 2nd ed., p. 503-538, 2012.
16. Tator K.B., Organic Coatings and Linings, in Corrosion: Fundamentals, Testing, and Protection, Vol 13A, ASM Handbook, ASM International, p.817833, 2003.
17. Anshuman S., Chuan Z., Austin C.Y., Ray K., Dane M., Ab initio and Thermodynamic Modelling of Alloying Effects on Activity of Sacrificial Aluminium Anodes, Elsevier Journal of Corrosion Science 2011, 5, p. 17241731.
18. Idusuyi N., Oluwole O. O., Aluminium Anode Activation Research, A Review International Journal of Science and Technology, 2012, 2(8), p. 561566.
19. Ulrich Z., Adhesion Testing, in Coatings Technology Handbook, 2nd Ed. Taylor and Francis, p. 26213, 2006.