Extraction and Characterization of Chitin from Nigerian Sources
Muhammed Tijani ISA1*, Alewo Opuada AMEH1, Joseph Owoicho GABRIEL1, and Kenneth Kennedy ADAMA2
1 Department of Chemical Engineering, Ahmadu Bello University Zaria, Zaria, Nigeria 810261
2 Physics Advanced Laboratory, Sheda Science and Technology Complex (SHESTCO), Abuja, Nigeria
E-mails: mtisaz@abu.edu.ng, alewooameh@yahoo.com savejoe4real@yahoo.com adama@shestco.org
* Corresponding author: Phone: +2348034536374
Abstract
The extraction and characterization of chitin from four sources of Nigerian origin was investigated. Chemical demineralization and deproteinization was done to obtain the chitin. Proximate analysis, XRD and SEM analysis were conducted on obtained chitins. The investigation revealed that the shrimp had the highest yield of chitin of 8.15%, crab, crayfish and periwinkle had yields of 7.8%, 2.88% and 0.44% respectively. The proximate analysis showed that chitin from shrimp had highest moisture and protein content of 8.70% and 4.16% respectively. Crayfish had the highest ash and fiber content of 7.20% and 6.98% respectively. Crab has the highest lipid content of 1.70%. The SEM analysis showed very uniform structure with a lamellar organization and less dense structure for chitin from shrimp and the surface of chitin from crayfish consists of fibers that form parallel thread networks. XRD analysis showed that chitin from shrimp was more crystalline than others.
Keywords
Chitin; Crustaceans; Demineralization; Deproteinization; Periwinkle.
Introduction
The term “chitin” is used to designate 1, 4-linked 2-acetamido-2-deoxy-β-D-glucose. It is a hard substance that makes up the exoskeleton of insects and crustaceans, which can also be obtained from other sources like fungi, mushrooms, worms, diatoms, etc. [1-5]. Chitin is the second most abundant natural polymer in nature after cellulose and like cellulose it functions as a structural polysaccharide [6].
Chitin and its derivatives have several applications in many field, these include, biomedical, food, emulsifying agent, wastewater treatment, biocatalysis, agriculture, textile and also paper industry [7,8].
It has been reported that isolation of chitin from different sources is affected by the source and also the percentage of chitin present in source where it is found varies according to the origin of the source [2-3]. Therefore, several works on the extraction and characterization of the chitin and its derivatives from different origins have been reported. Limam et al. in [9] reported on the extraction and characterization of chitin and chitosan from two species of crustacean of Tunisian origin. Also, Al-Sagheer et al. in [10] produced chitin from Arabian Gulf crustaceans’ sources to determine the protein content in chitin. Abdou et al. in [3], reported the production of chitin and its derivative from crustacean of Egyptian origin. Yildiz et al. in [11] reported the extraction and characterization of chitin and chitosan from Mediterranean crab.
Despite all these reported work, little is available on the extraction and characterization of chitin from crustacean of Nigerian origin. Sources of chitins are highly available in Nigeria and are abundant in the rural areas of Nigeria [12,13]. These materials litter the banks of rivers constituting environmental pollution because they are underutilized. More so, the products of the crustacean are also discarded after processing and these are valuable sources of chitin, which can be further, processed into chitosan.
The aim of the research was to quantity of chitin in some selected crustaceans and periwinkle of Nigerian origin. This will give guide to researchers as to where source chitin for chitosan synthesis.
Material and Method
Chitin Extraction
Crab, crayfish, periwinkle and shrimp of Nigeria origin were obtained and taken to the Department of Biological Science, Ahmadu Bello University, Zaria, Nigeria for identification. The shells of these different species were scraped free of loose tissue, wash, dry, and ground to pass through a 250 micro meter sieve. Chitin was extracted from100g of the size reduced shells by demineralization and deproteinization.
Demineralization was carried out at room temperature using 1 M hydrochloric acid. The process was repeated several times until evolution of gas ceased and the number of baths and duration were dependent of shell type. The crab shell took longer time and number of bath. The resulting solids from different samples were washed with distilled water until neutral pH. The demineralized samples were dried in the oven at 60°C to constant weights.
Deproteinization was carried out on each sample by heating in 1 M sodium hydroxide solution in a beaker 100°C. This treatment was repeated several times and absence of protein was indicated by the absence of colour of the medium at the end of the last treatment. The samples were washed with distilled water up to neutrality, after which the samples were dried. The quantity of the chitin was calculated after this process.
Proximate Analysis of Chitin
Proximate analysis was conducted to determine the ash content, moisture content, lipid content, protein content and carbohydrate content of chitin. The analyses were carried out using AOAC standards [14].
Structural Analysis of Chitin
The X-ray diffraction of the samples was conducted using PAN analytical X’ Pert PRO MPD X-ray diffraction system PW3040/60 machine at Sheda Science and Technology Complex (SHESTCO), Abuja, Nigeria. The prepared samples were prepared and held on a sample holder and beams of electron passed through. The intensity was measured at Bragg’s 2θ angle. This was done to establish the degree of crystallinity of the various chitin samples. The morphology of the chitin samples were visualized using a scanning electron microscope (JEOL 6400) at SHESTCO.
Results
The calculated composition of the four samples after demineralization and deproteinization showing chitin yield is presented in Table 1.
Table 1. Percentage composition of Shrimp, Crab, Crayfish and Periwinkle
|
Chitin source |
% CaCO3 |
% Protein |
% Chitin |
|
Shrimp shells |
76.80 |
15.05 |
8.15 |
|
Crab shells |
78.70 |
13.50 |
7.80 |
|
Crayfish |
66.80 |
30.32 |
2 .88 |
|
Periwinkle shells |
97.5 |
2.06 |
0.44 |
The proximate analysis of the chitin samples are presented in Table 2.
Table 2. Proximate Analysis of Chitin from Nigerian Sources
|
Parameters (%) |
Shrimp |
Crab |
Crayfish |
|
Moisture |
8.70 |
6.10 |
6.30 |
|
Ash |
5.60 |
3.40 |
7.20 |
|
Fiber |
6.12 |
5.70 |
6.98 |
|
Protein |
4.16 |
2.60 |
2.46 |
|
Lipid |
1.30 |
1.70 |
1.20 |
|
Carbohydrate |
74.12 |
80.50 |
75.86 |
Figure 1, presents the super imposed XRD pattern of chitin from shrimp, crab and crayfish. Figures 2, 3 and 4 show the SEM micrograph of the chitin from shrimp, crab and crayfish.
|
|
|
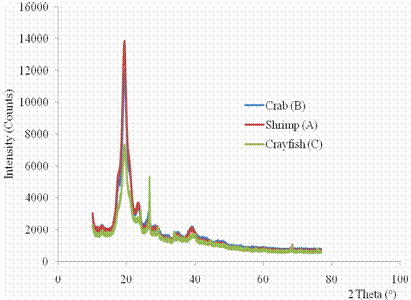
Figure 1. Superimposed X-ray diffraction patterns of shrimp (A), crab (B) and crayfish (C) |
|
|
|
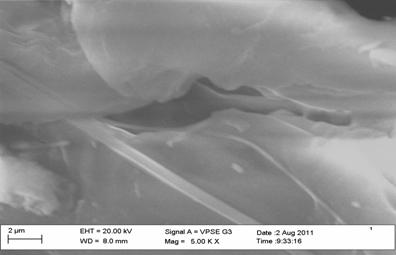
Figure 2. Scanning electron micrograph of dry surface of chitin from shrimp |
|
|
|
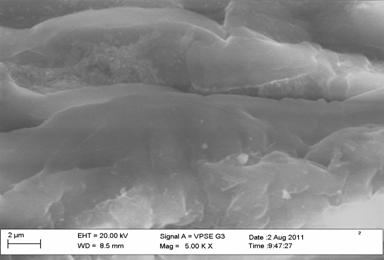
Figure 3. Scanning electron micrograph of dry surface of chitin from crab |
|
|
|
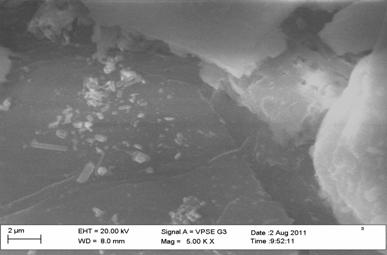
Figure 4. Scanning electron micrograph of dry surface of chitin from crayfish |
Discussions
The result of chitin composition presented in Table 1 showed that the percentage of inorganic matter (CaCO3) was lowest in crayfish and highest in periwinkle. Crab contains higher inorganic material than shrimp. In the work of Abdou et al., [3] pink shrimp, crab shells and crayfish shells were reported to have CaCO3 content of 42.26, 66.58 and 63.94 % respectively. The CaCO3 obtained in this work was higher than the reported; compositions of sources are reported to vary from region to region [2]. The periwinkle shells also have the lowest protein content as evaluated after deprotenization. The Crayfish had the highest protein content which is an indication that it contained more organics than the other samples. The crayfish flesh could not be removed completely from the shell because of its small size, therefore, probably resulting to the presences of high protein content. The chitin content of the crustacean shells ranged between 0.44-8.15%. The shrimp shells had the highest chitin content while the periwinkle had the least. This is evident considering the inorganic content of the periwinkle when compared with the other samples. Shahidi and Synowieecki, in [15] reported chitin composition for crab and shrimp to be between 17-32.2%. However, Abdou, et al., in [3] reported chitin yield of 23.72, 16.73 and 20.60% for pink shrimp, crab shells and crayfish respectively. Wide deviation was observed in this work when compared with reported values. Probably origin and method of extraction would have affected the yields. Due to the extremely low content of the chitin in the periwinkle shell, no further analysis was carried out on it.
Chitin characterization results in this study, which consists of the proximate analysis as given in Table 2, showed that the moisture content of chitin from shrimp was higher than the chitin from crab and crayfish. The differences in moisture content could probably be attributed to amount of moisture absorbed by difference chitin samples after synthesis. The high value of ash content in chitin from crayfish indicates a high mineral content in it, than the chitin from shrimp and crab after demineralization. The analysis also indicated that the chitin from crayfish had highest value of fiber content and crab had the least. This could be that chitin from crayfish has high cellulose than those from shrimp and crab. The high protein content of chitin from shrimp was an indication that it has more nitrogenous substances than chitin from crab and crayfish. Probably the removal of protein during deproteinization was more effective in crab and crayfish than shrimp. The lipids and carbohydrate content of chitin from crab was also determined to be higher than that of chitin from shrimp and crayfish.
XRD analysis was carried out on the chitin samples to verify the degree crystallinity of the isolated chitin. The super imposed XRD patterns of the chitin of shrimp (A), crab (B) and crayfish (C) Figure 1, showed five sharp peaks at 200, 240, 260, 340, 390 2θ angles but at different intensities, with the peaks of the shrimp having the highest intensity at 2θ angle of 200. The XRD profile of the chitin of shrimp exhibits well resolved and intense peaks, while broad diffuse scattering and less intense peaks are found for crab and crayfish. This indicates that shrimp chitin is more crystalline polymorph than crab and crayfish.
The surface morphology of chitin was observed using SEM, Figure 2 and 3 showed that the surface of chitin of shrimp and crab have scarcely fibrillar material and granular structure. The surface of chitin from crayfish consists of fibers that form parallel thread networks as shown in Figure 4. A very uniform structure with a lamellar organization and less dense structure was observed clearly for chitin from shrimp, whereas the surface of chitin of crab and crayfish appeared less crystalline and different from each other.
Conclusions
Chitin was extracted from four different sources (crab, shrimp, crayfish and periwinkle) of Nigerian origin with shrimp having the highest yield of chitin of 8.15% and periwinkle with least yield of 0.04%. XRD analysis indicated that prominent peaks for the chitins appeared at the same 2θ angles but at different intensity, indicating slightly different degree of crystallinity of the chitin from different sources. SEM analysis indicated a very lamellar organization and less dense structure of chitin from shrimp than crab and crayfish.
Acknowledgements
The authors wish to thank the, Sheda Science and Technology Complex for allowing the use of their X-Ray Diffraction machine and scanning electron microscope
References
1. Blackwell J., Walton A.G., Chitin In: Biopolymers, New York, Academic Press, 1973, p.474-489.
2. Muzzarelli R.A.A., Some modified chitosan and their niche applications, In Chitin Handbook, by Muzzarelli R.A.A., Peter M.G. (ed). European Chitin Society, Italy, 1997, p.47-52.
3. Abdou E.S., Nagy K.S.A., Elsabee M.Z., Extraction and characterization of chitin and chitosan from local sources, Bioresources Technology, 2008, 99, p.1359-1367.
4. Nessa F., Shah M.M., Asaduzzaman M., Roy S.K., Hossain M.M., Jahan M.S., A process for the preparation of chitin and chitosan from prawn shell waste, Bangladesh J. Sci. Ind. Res., 2010, 45(4), p. 323-330.
5. Inmaculada A., Marian M., Ruth H., Inés P., Beatriz M., Niuris A., Gemma G., Ángeles H., Functional characterization of chitin and chitosan, Current Chemical Biology, 2009, 3, p. 203-230.
6. Dutta P.K., Dutta J., Tripathi V.S., Chitin and chitosan: Chemistry, properties and applications, J. of Sc. and Ind. Res., 2004, 63, p. 20-31.
7. Freier T., Koh H.S., Kazazian K., Shoichet M.S., Controlling cell adhesion and degradation of Chitosan films by N-acetylation, Biomaterials, 2005; 26(29), p. 5872-5878.
8. Kalut S.A., Enhancement of degree of deacetylation of chitin in chitosan production, B. Chemical Engineering, Universiti Malaysia Pahang, 2008, p. 14-15.
9. Limam Z., Selmi S., Sadok Saloua, El Abed A., Extraction and characterization of chitin and chitosan from crustacean by-products: Biological and physiochemical properties, African journal of biotechnology, 2011, 10(4), p.640-647.
10. Al-Sagheer F.A., Al-Sughayer M.A., Muslim S., Elsabee M.Z., Extraction and characterization of chitin and chitosan from marine sources in Arabian Gulf, Carbohydrate Polymers, 2009, 77(2), p. 410-419.
11. Yildiz B., Sengul B., Ali G., Levent I., Seval B.K., Soner C., Habil U.K., Chitin –chitosan yields of fresh water crab (Potamon potamios, Olivier 1804) shell, Pak. Vet. J., 2010, 30(4), p. 227-231.
12. Amos T.T., Sustainable poly-culture fish production in Kano state of Nigeria: An economic analysis, PhD Thesis, Department of Agricultural Economics, Ahmadu Bello University, Zaria, Nigeria, 2000, p.11-15.
13. Amos T.T., Production and productivity of crustacean in Nigeria, J. Soc. Sci., 2007, 15(3), p. 229-233.
14. AOAC, Official methods of analysis, 15th ed.; Association of Official Analytical Chemists, Washington DC, 1990.
15. Shahidi F., Synowieck J., Isolation and charactrization of nutrients and value-added products from snow crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing discards, Journal of Agricultural Food Chemistry, 1991, 39, p. 1527-1532.