The Inhibitive Potential of Arecaceae Extract on the Corrosivity of Aluminium based Matrix Composite and Medium Carbon Steel in Different Media
Paul Aondona IHOM*, Francis AYENI, Suleiman MOHAMMED, and Solomon OLA
National Metallurgical Development Centre, Jos PMB 2116 Zaria Road,
Jos Plateau State
E-mails: paulihom@yahoo.co.uk *; ayeni71@yahoo.com ; suleimoh@yahoo.com ; olasolomon@yahoo.com
* Corresponding author: Phone: +2348035813571
Abstract
In this research study, the inhibitive potential of Arecaceae extract on the corrosivity of aluminium based matrix composite and medium carbon steel in different media have been undertaken. The composite and the carbon steel were exposed in 0.5M NaOH and 0.5M HCl solutions as the control solutions. The Arecaceae extract from the fruit juice was added in volume percentages of 5%v/v and 10%v/v. The exposure time covered 12 days, and the monitored temperature range during the exposure time was 25-39°C. The result indicated that the most corrosive media were those without the Arecaceae inhibitor; where corrosion rate ranged from 0.106mpy to 0.409mpy. The medium carbon steel did not show any sign of corrosion in the 0.5M NaOH solution. Arecaceae inhibitor addition of 5%v/v gave optimum corrosion rate reduction; the corrosion rate was in the range of 0.018mpy to 0.199mpy. Although the lowest range of 0.013mpy to 0.192mpy was obtained with 10%v/v of Arecaceae, however on the overall performance the 5%v/v of Arecaceae was better. The inhibition of corrosion by Arecaceae was as a result of adsorption and phase layers on the surface of the metals.
Keywords
Inhibitor; Arecaceae; Corrosivity; Media; Aluminium Composite; Medium Carbon Steel.
Introduction
New materials are emerging on daily basis. Recent research is targeted at developing corrosion resistant materials with inherent corrosion resistant superior to existing materials. These new materials are sometimes called advanced materials [1,2], while the research for their development persist, effective ways must be device for the preservation of the degradation of materials in current usage. Most engineering materials degrade in one medium or another.
Not all materials have the ability to resist the attack of the environment they found themselves in. They may be resistant in one environment, while the opposite may be the case in another environment. When this happens the need always arises to protect the material from deterioration, and there are several methods of corrosion prevention [3]. The use of inhibitors is one of the methods. Through the use of the correct inhibitor an aggressive and destructive environment can be made to be less destructive or friendly to the material [4]. Inhibitors tend to ameliorate the destructive tendency of an aggressive environment. There are different types of inhibitors some of them are organic while others are inorganic. A good example of the usefulness of inhibitors can be seen in automobiles, where radiator coolants also serve as inhibitors in preventing engine and radiator rusting and corrosion. The products of the rust in the radiator normally result into overheating of the engine; the radiator coolant prevents this by inhibiting corrosion. Antirust inhibitors are also used on car bodies before subsequent and final coating work on the car [5, 6]. The importance of inhibitors to materials corrosion prevention is enormous. Plant extracts have of recent become a centre stage for research work for the development of new inhibitors [7-10].
This work examines the corrosion inhibitive potentials of a wild date palm commonly used as ornamental tree from the palm family of Arecaceae or Palmae. This specie is closely related to phoenix dactylifera and phoenix sylvestris which are used for the production of date sugar. The date palm grows about 23 meters [75 feet] tall. Its stem, strongly marked with the pruned stubs of old leaf bases, terminates in a crown of graceful; shining pinnate leaves about 5 meters [16 feet] long floral spikes branch from the axils of leaves that emerged the previous years. Male and female flowers are borne on separate plants. Under cultivation the female flowers are artificially pollinated. The date is a one seeded fruit, or berry usually round to oblong but varying much in shape, size, colour, quality, and consistency of flesh, according to the conditions of culture. More than 1000 dates may appear on a single bunch weighing 8kg [18 pounds] or more [11]. No literature so far exist to the knowledge of the authors on the corrosion inhibitive potentials of the juice extracts from the fruits of this plant. No evidence that this study has been done by any one. It is a completely new investigation although other plants here have been investigated [4, 7-10]. The chemical composition of the fruits from Arecaceae is unique and may provide a fantastic inhibition to corrosion of metals even in highly aggressive medium. The dried fruit is more than 50 percent sugar by weight and contains about 2 percent each of protein, fat and mineral matter.
The aim of the research was to investigate the potentials of Arecaceae fruit juice extract as corrosion inhibitor, to assess the degree of protection given to some metals in some aggressive medium and to note the pH of the various media used. This research will also assess the rate of corrosion of the metals in all the media, so as to determine the potential of the Arecaceae fruit juice extract as an inhibitor.
Material and Method
Materials
The materials used for the research work were, carbon steel, aluminium 2.0% glass particulate reinforced composite material, 0.5M HCl, 0.5MNaOH, acetone Arecaceae fruit juice extract, and distilled water. In table 1-3 present the chemical compositions of some materials used. Figure 1 shows the plate of the Arecaceae tree.
Table 1. Chemical Composition of the Carbon Steel used
|
Element |
Element |
% |
Element |
% |
Element |
% |
Element |
% |
|
|
C |
0.306 |
Ni |
0.0085 |
Zn |
0.012 |
Ti |
0.001 |
La |
0.0066 |
|
Si |
0.172 |
V |
0.0047 |
As |
0.0043 |
Nb |
<0.0030 |
Fe |
98.7 |
|
Mn |
0.6 |
W |
0.018 |
Mo |
<0.0020 |
Bi |
<0.0020 |
|
|
|
P |
0.029 |
Pb |
<0.0030 |
Al |
0.012 |
Ca |
0.0099 |
|
|
|
S |
0.024 |
B |
<0.005 |
Cu |
0.026 |
Ce |
0.0041 |
|
|
|
Cr |
0.027 |
Sn |
<0.001 |
Co |
0.0076 |
Zr |
<0.0015 |
|
|
Table 2. Chemical Composition of the Composite used
|
Composition |
Al |
Glass particles |
|
% |
98.0 |
2.0 |
Table 3. Chemical Composition of Arecaceae Fruit Juice Extract
|
Composition |
Sugar |
Protein |
Fat |
Mineral Matter |
Water |
|
Wt. % |
>50 |
2 |
2 |
2 |
Balance |
|
|
|
Figure 1. The Arecaceae Tree from which the Fruits were taken |
Equipments
The equipments used, were: electronic digital weighing balance, steel brush, beakers, glass flasks, thread, stand and holder, and digital pH meter made by Easy Way Medical England, model PHS –25.
Methods
The work started with specimen preparation. Test coupons were cut from aluminium 2.0% glass reinforced composite and medium carbon steel, the coupons were cleaned using acetone, and a steel brush to remove dirt and scales. They were then washed and dried using a hand air blower; the initial weight of each of the specimen was taken using an electronic digital weighing balance.
The Arecaceae plant juice was extracted by plucking the ripped fruits and squeezing them to remove the juice. The extracted juice was then filtered using a sieve of aperture ~38 microns. The filtrate was put in a container and covered for later use.
Two media were prepared; one was 0.5M HCl and the other 0.5M NaOH solutions. These solutions were then transferred into other containers of capacity 500 ml each. Two of the containers contain 0.5M NaOH solution and 0.5M HCl solution each, in the remaining containers, the filtrate from Arecaceae fruit juice was introduced in varying composition of 5%v/v and 10%v/v. Specimens of the aluminium composite and the medium carbon steel were then suspended in the various media to cover the measurement days of 4 days, 8 days and 12 days. A specimen was removed; one each for every metal, on every measurement day, it was washed using acetone and metal brush and then finally rinsed in distilled water. It was then dried using an air blower before weighing using an electronic digital weighing balance. The weight loss was then determined by subtracting the final weight from the initial weight of the test coupons. Figure 2 shows the experimental set up of the work. The fast weight loss on the composite coupons in the two media without inhibitor determined the duration of the study. The study is however ongoing and the experiment will be extended for 3 months to see the effect of the inhibitor over an extended period of time. The experiment was conducted within the temperature range of 25°C-39°C.
|
|
|
Figure 2. The Experimental Set up of the Work |
Results and Discussion
Tables 4-7 show the results of the weight loss (in grams) of the metals in the various media, covering 4 to 12 days.
Table 4. Effect of Arecaceae on the weight loss (in grams) of Medium Carbon Steel in HCl solution (500 ml 0.5 M)
|
Media |
% v/v Arecaceae |
4 days |
8 days |
12 days |
|
1 |
0 |
0.704 |
1.145 |
1.304 |
|
2 |
5 |
0.128 |
0.238 |
0.495 |
|
3 |
10 |
0.084 |
0.643 |
0.912 |
Table 4 shows the effect of Arecaceae on the weight loss of medium carbon steel in 0.5M HCl solution. The table showed that the medium containing medium carbon steel without Arecaceae extract has the highest weight loss. After 4 days the weight loss was 0.704g, the weight loss climax to 1.304g after 12 days. When 5%v/v of Arecaceae was introduced after 4 days the weight loss dropped to 0.128g and after 12 days it dropped to 0.495g as against 1.304g in the medium without Arecaceae. When 10%v/v of Arecaceae was introduced on the 4th day the weight loss of the medium carbon steel dropped to 0.084g as against 0.704g in the medium without Arecaceae and after 12 days it dropped to 0.912g as against 1.304g in the medium without Arecaceae. A similar observation had been made by some researchers [12,13].
Table 5, shows the effect of Arecaceae on the weight loss of aluminum composite in 0.5M HCl the table showed that the medium without Arecaceae has the highest weight loss 0.433g on the 4th day and 0.46g on the 12th day.
Table 5. Effect of Arecaceae on the weight loss (in grams) of Aluminium Composite in HCl solution (500 ml 0.5 M)
|
Media |
% v/v Arecaceae |
4 days |
8 days |
12 days |
|
1 |
0 |
0.433 |
0.411 |
0.546 |
|
2 |
5 |
0.34 |
0.35 |
0.448 |
|
3 |
10 |
0.31 |
0.404 |
0.460 |
The medium that had 5%v/v of Arecaceae introduced in 0.5M HCl solution showed that the weight loss reduced to 0.34g on the 4th day and 0.448g on the 12th day. When 10%v/v of Arecaceae was added to 0.5M HCl, solution the weight loss dropped to 0.31g on the 4th day and 0.460 on the 12th day.
Table 6 shows the effect of Arecaceae on the weight loss of medium carbon steel in 0.5M NaOH solution. The result in the table has shown that for the duration of the experiment there was no weight loss in any of the three media. This is an indication that medium carbon steel of the composition shown in Table 1 has corrosion resistance in 0.5M NaOH solution within the exposure time of 12 days this agrees with some literatures reviewed [13,17]. Table 7, shows the effect of Arecaceae on the weight loss of aluminium composite in 0.5M NaOH solution.
Table 6. Effect of Arecaceae on the weight loss of Medium Carbon Steel in NaOH solution (500 ml 0.5 M)
|
Media |
% V/V Arecaceae |
4 days |
8 days |
12 days |
|
1 |
0 |
0.0 |
0.0 |
0.0 |
|
2 |
5 |
0.0 |
0.0 |
0.0 |
|
3 |
10 |
0.0 |
0.0 |
0.0 |
Table 7. Effect of Arecaceae on weight loss of Aluminium Composite in NaOH solution (500 mol 0.5 M)
|
Media |
% v/v Arecaceae |
4 days |
8 days |
12 days |
|
1 |
0 |
1.600 |
1.134 |
2.926 |
|
2 |
5 |
0.772 |
0.828 |
0.881 |
|
3 |
10 |
0.751 |
0.84 |
0.849 |
The environment was really aggressive to the aluminium composite, on the 4th day of exposure the weight loss rose to 1.6g and climbed to 2.926g on the 12th day. This was the first medium which only contains 0.5M NaOH solution. In the second medium which contains 5%v/v of Arecaceae the weight loss dropped to 0.772g on the 4th day and 0.881 on the 12th day. In the third medium which contains 10%v/v of Arecaceae the weight loss dropped to 0.75g on the 4th day as against 1.600g in the medium without Arecaceae and 0.849 on the 12th day as against 2.926 in the medium without Arecaceae.
The results from Tables 4-7 have clearly shown that Arecaceae have the potentials to be used as an inhibitor in reducing the effect of corrosion of medium carbon steel and aluminium matrix based composite material [Al 2% reinforced glass particulate composite] in aggressive media like 0.5M NaOH and 0.5M HCl solutions. The above observations have been reported by various authors [13-17] in their work on corrosion. The mechanism of the inhibitive property of inhibitors has been described by Fontana [17]. According to the author an inhibitor is a substance that, when added in small concentrations to an environment decreases the corrosion rate. In a sense, an inhibitor can be considered as a retarding catalyst. Inhibition is not completely understood because of these reasons, but it is possible to classify inhibitors according to their mechanism and composition. The inhibitor used in this work is an adsorption type inhibitor and the inhibitive action of the Arecaceae was through adsorption, this is the common inhibition mechanism exhibited by most organic compounds. The Arecaceae is made up of more than 50% sugar or carbohydrate, 2% protein and 2% fat these compounds adsorb on the metal surface and suppress metal dissolution and reduction reactions. Thereby reducing the weight loss of the metal, this happens because the Arecaceae [adsorption inhibitor] affected both the anodic and cathodic processes. As the concentrations reduces with time the weight loss increases indicating an increase in anodic process. Concentration and temperature is therefore an important factor in the effectiveness of an inhibitor this has been corroborated by several authors [13-17]. Corrosion inhibitor reference table [17] has shown that 5% inhibitor addition provides optimum result. This has also been observed in Tables 4-7.
Figures 3-5 shows the corrosion rate in mPy of medium carbon steel and Al 2.0% glass particulate reinforced composite in 0.5M NaOH, 0.5M HCl solutions, and in media containing 5%v/v of Arecaceae and 10%v/v of Arecaceae.
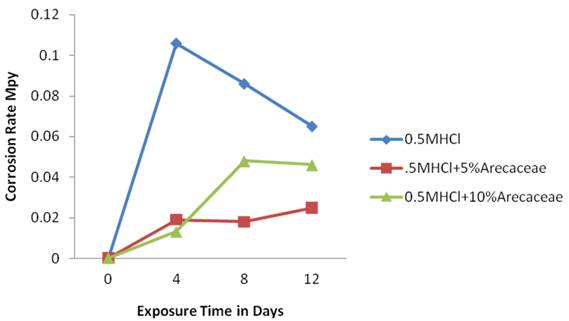
Figure 3 shows the corrosion rate of medium carbon steel in three media. The control medium [O.5M HCl] had the highest corrosion rate as can be seen by the position of the plot which is higher up.
|
|
|
Figure 3. The Effect of Arecaceae Extract on the Corrosion Rate of Medium Carbon Steel in 0.5M HCl Solution |
This is followed by the medium with 10%v/v of Arecaceae in 0.5M HCl. The medium with 5%v/v of Arecaceae in 0.5M HCl has the lowest corrosion rate compared to the two other plots. This result agrees with corrosion inhibitor reference table which showed that optimum result is normally obtained with 5% inhibitor and below. In figure 3 of the plot with Arecaceae [inhibitor] it can be seen that the corrosion rate reduced drastically on the 4th day and then increased as the exposure time increased indicating a reduction in the Arecaceae concentration. This trend is common in corrosion control using inhibitors [16]. Fontana [17] had explained that “it is important to remember that inhibitors are specific in terms of metal, environment, temperature, and concentration range. It is important to use enough inhibitor, since many inhibiting agents accelerate corrosion, particularly localized attack such as pitting, when present in small concentrations. Hence, too little inhibitor is less desirable than none at all. To avoid this possibility, inhibitors should be added in excess and their concentrations checked periodically”.
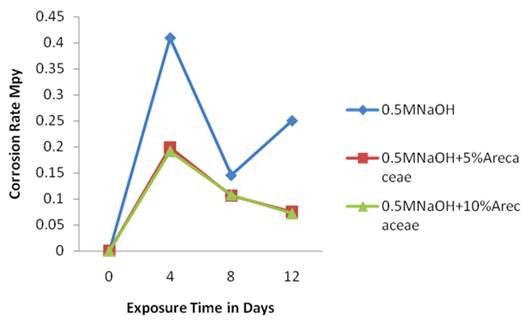
Figure 4 shows the corrosion rate of Al 2.0 % glass composite in three media. The plots of the corrosion rates showed the control medium [0.5M HCl] at the top followed by the medium with 10%v/v of Arecaceae and then the medium with 5%v/v Arecaceae is at the bottom, this trend was also observed in figure 3.
|
|
|
Figure 4. The Effect of Arecaceae Extract on the Corrosion Rate of Aluminum Matrix Based Composite in 0.5M NaOH Solution |
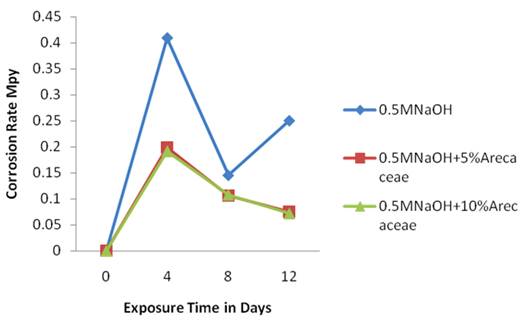
Figure 5 shows the corrosion rate of Al 2.0% glass composite in three media. The plots of the corrosion rates against exposure time showed the control medium [0.5M NaOH] at the top followed by the medium with 5%v/v of Arecaceae in 0.5M NaOH which is not clearly distinguished from 10%v/v of Arecaceae in 0.5M NaOH which followed at the bottom of the plots.
|
|
|
Figure 5. The Effect of Arecaceae Extract on the Corrosion Rate of Aluminum Matrix Based Composite in 0.5M NaOH Solution |
Both Figures 4-5 has shown that the addition of Arecaceae extract to the two corrosives has led to the reduction of the corrosion rate of medium carbon steel and Al 2.0% glass reinforced composite. Inhibition can be caused by both adsorption and phase layers on the metal surface.
The inhibitor mechanism, irrespective of the amount adsorbed by the surface consists in changing the electro-physical properties of surface atoms by the by the donor-acceptor inhibitor metal reaction, rather than in screening the metal surface from the corrosive environment [18]. An international conference on corrosion inhibition was held during May 1983 and this statement was made: “Evidence presented during recent years indicates that many organic and inorganic inhibitors become effective though interaction with one of several corrosion products to form a new protective phase rather than by absorption on the metal surface” [19]. The above explanations seek to explain the inhibition behaviour of Arecaceae in the two media one of which is alkaline and the other acidic. Given the behaviour of Arecaceae in figures 3-5 it can be considered as an inhibitor.
Table 8 presents the pH values of the various media which the two metals were exposed to. The inhibitor [Arecaceae] itself had a pH value of 3.87 which is acidic in nature. The 0.5M HCl had a pH value of 0.53, the 0.5M NaOH had a pH of 12.78, 5%v/v of the inhibitor in 0.5M HCl had a pH value of 1.15, 10%v/v inhibitor in 0.5M HCl had a pH value of 2.10, 5% v/v inhibitor in 0.5M NaOH had a pH value of 10.54 and 10%v/v inhibitor in 0.5M NaOH had a pH value of 10.42. The results showed that the 0.5M HCl is extremely acidic and the 0.5M NaOH is extremely alkaline. The addition of Arecaceae which has a pH value of 3.87 to 0.5M HCl in the percentages 5% and 10% changed the pH values to 1.15 and 2.10.
Table 8. PH Values of the Various Media
|
No. |
Medium |
PH value |
|
1. |
Inhibitor [Arecaceae] |
3.87 |
|
2. |
0.5M HCl [control] |
0.53 |
|
3. |
0.5 M NaOH [control] |
12.78 |
|
4. |
5%v/v inhibitor in 0.5M HCl |
1.15 |
|
5. |
10%v/v inhibitor in 0.5M HCl |
2.10 |
|
6. |
5%v/v inhibitor in 0.5M NaOH |
10.54 |
|
7. |
10%v/v inhibitor in 0.5M NaOH |
10.42 |
The addition of Arecaceae to 0.5M NaOH in the percentages 5-10% changed the pH values to 10.54 to 10.42. The addition of Arecaceae to the acidic medium reduced the acidic of the medium. The addition of Arecaceae to the alkaline medium reduced the alkalinity from 12.78 to 10.54 and 10.42.
In all the media where the pH was adjusted as a result of the addition of the Arecaceae extract, saw the reduction in corrosion rate. Medium carbon steel and Al 2.0% glass reinforced composite had highest corrosion rate in 0.5M HCl and 0.5M NaOH media. Medium carbon steel was however corrosion resistant in 0.5M NaOH solution within the time of exposure, the addition of Arecaceae extract to the medium did not change the status quo. From results seen in Table 8 and in Figures 3-5, pH values can be said to have an effect on corrosion rate of medium carbon steel and Al 2.0% glass reinforced composite.
The above premise is true because potential - pH plots called pourbaix diagram have been used to predict corrosion in metals in a given environment [3, 17] it has been explained that pH values tend to increase the critical anodic current density and usually have relatively little effect on the Primary passive potential and passive dissolution rate. The pH value of the environment affects the corrosion behaviour of a material that explains why some materials are attacked in dilute solutions and not in concentrated solutions and vice-versa [3, 17].
Conclusions
The study had undertaken the critical examination of the potentials of Arecaceae extract as an inhibitor for reducing the corrosion rate of medium carbon steel and Al/2.0% glass reinforced particulate composite. Based on the empirical result with Arecaceae extract as the inhibitor in 0.5M HCl and 0.5M NaOH solutions the following conclusions have been deduced:
· Within the monitored environmental temperature range of 25°C to 39°C the medium carbon steel and Al/2.0% glass reinforced particulate composite corroded in 0.5M HCl and 0.5M NaOH solutions within the exposure time of the experiment.
· The study had shown that 5%v/v of Arecaceae used as inhibitor produced optimum result for the corrosion rate reduction in the two media within the exposure time and temperature range. The inhibition action is by adsorption and phase layer formation on the metal surface, which consists in changing the electro-physical properties of the surface atom by the donor-acceptor inhibitor/metal reaction, rather than in screening the metal surface from the medium.
· Finally the study has clearly proven that Arecaceae extract can be used as an inhibitor in 0.5M HCl and 0.5M NaOH solutions for the reduction of corrosion rate of medium carbon steel and Al 2.0% glass reinforced particulate composite.
Acknowledgements
The authors acknowledge the contributions of the National Youth Service corp. members attached to the Department of Metallurgy of the National Metallurgical Development Centre Jos. We thank you Mr. Vitalis U. and Mr. Osundo K. very much for taking your time to prepare the materials that were used for the research work.
References
1. Gandhi M.V., Thompson B.S., Smart Materials and Structures, First Edition, London, Chapman and Hall, 1992, p. 1-34.
2. Ihom A.P., New Materials for a New Technological Era, as Part of JICA Action Plan, 2009, p. 1-5.
3. Ihom A.P., Nyior G.B., Nor I.J., Segun S., Ogbodo J., Evaluation of the corrosion resistance of Aluminium Alloy Matrix/ 2.5% Particulate Glass Reinforced Composite in Various Media, International J. of Science and Tech. 1(10), 2012, p.560-568, Available at: http://www.journalofsciences-technology.org/archive/2012/oct_vol_1_no_10/8188271348145897.pdf (accessed 20/10/2010).
4. Yawas D.S., Effect of Some Inhibitors on the Corrosion Behaviour of Mild Steel Reinforced Concrete Structures, Book of abstracts for Nigerian Corrosion Association National Conference, AGM Held in Jos-Nigeria, 2005.
5. Madu P.C., Asoegwu S.N., Ogbonna A.I., Onuchukwu A.I., The Automobile Cooling System: the Effect of Antirust Agents, Journal of Corrosion Science and Technology, 2004, 1(1), p. 92-94.
6. Ihom A.P., Alabi O.O., Nyior G.B., Ogbodo J., Anbua E.E., Evaluation of the Corrosion Resistance of Reinforcement Steel Rod in NMDC Borehole Water, International J. of Sci. and Eng. Research, 3(12), 2012, p.1-9.
7. Olusegun K.A., Oforka N.C., Ebenso E.E., The inhibition of Mild Steel Corrosion in an Acidic Medium by Fruit Juice of Citrus Paradisi, Journal of Corrosion Science and Technology, 2004, 1(1), p. 75-78.
8. Kolo A.M., Onuchukwu A.I., Adamu H.M., Bawa M.A., Lawsonia Inermis and Parkia biglobosa Extracts as Corrosion Inhibitors of Mild Steel in HCl, Nigerian Corrosion Association Book of Abstracts for the Annual General Meeting and National Conference, 2005.
9. Aku S.Y., Oloche O.B., Yawas D.S., Investigation of Non-toxic Plant Extracts (Acacia Nilotica pod and khaya segegaleasis) as Corrosion Inhibitors of Low Carbon Steel in Hydrochloric Acid Pickling Solution, Nigerian Corrosion Association Book of Abstracts for the National Conference and Annual General Meeting, 2005.
10. Adeyemi O.O., Oluwafemi S.O., Adebogun A.A., Inhibition of the Corrosion of Mild Steel in Hydrochloric Acid Solution by Naturally Occurring Substance, Nigerian Corrosion Association Book of Abstracts for the National Conference and AGM, 2005.
11. Date Palm, Encyclopedia Britannica Ultimate Reference Suite, Chicago: Encyclopedia Britannica, Available at: www.encyclopedia.Britannica.com (accessed 20/10/2010).
12. Ita B.I., The Study of Corrosion Inhibition of Mild Steel in Hydrochloric Acid by a-Vanillin and a-Vanillin Hydrazine, Journal of Corrosion Science and Technology, 2004, 1(1), p. 40-47.
13. Abiola O.K., Adsorption of Methionine on Mild Steel, Nigerian Corrosion Association Book of Abstracts for the National Conference and AGM, 2005.
14. Oguzie E.E., Okolue B.N., Ogukwe C.E., Onuchukwu A.I., Unaegbu, C., Studies on the Inhibitive Action of Methylene Blue Eye on Aluminum Corrosion in KOH Solutions, J.of Sc. & Tech., 2004, 1(1), p. 89-91.
15. Ebenso E.E., Umoren S., Jackson E., Ekpe U.J., Synergistic Effect of Halide Ions on the Corrosion Inhibition of Aluminum and Mild Steel in Acidic Medium by Gum Arabic, J. of Cor. Sc. & Tech., 2004, 1(1), p. 84-85.
16. Adeyemo O.O., Effect of Temperature and Concentration on Protective Action of 5-Membered Heterocycles on Acid Corrosion of Brass, Nigerian Corrosion Association Book of Abstracts for the National Conference and AGM, 2005.
17. Fontana M.G., Corrosion Engineering, 4th Edition, McgrawHill Book Company, 1987, p. 282-369.
18. Rosenfeld I.L., New Data on the Mechanism of Metals Protection with Inhibitors, Corrosion, 1981, 37, p. 371-377.
19. Ayeni F.A., Madugu I.A., Sukop P., Ihom, A.P., Alabi O.O., Okara R., Effect of Aqueous Extracts of Bitter Leaf Powder on the Corrosion Inhibition of Al-Si Alloy in 0.5M Caustic Soda Solution, J.of Min. And Matls. Characterisation and Engrg., 2012, 11, p. 667-670.