Kinetics of Adsorption of Nickel Ion on Kankara Kaolinite
Lawrence
Department of Chemical Engineering,
E-mails: osas1law@yahoo.com *; Simongabs@gmail.com ; abiolaidowu@yahoo.com ; bawasolomon13@yahoo.com
* Corresponding author: Phone: +447404710947
Abstract
In this work, the kinetics and dynamics of nickel ion adsorption on calcined kaolinite clay were studied. The Kinetic models (k1=0.025, k2=0.00065 and ki=2.089 g/(mg·min) were evaluated in order to identify potential adsorption mechanisms. The kinetic data were best represented by the pseudo first order model (R2=0.959) and the adsorption process is favored by decrease in pH i.e. Acidic condition (below 5.0). The Langmuir model was found to best suit the adsorption isotherm of nickel ion on clay than the Freundlich model. The maximum adsorption capacity of Kankara kaolinite was found to be 97.68% at a temperature of 50°C and pH of 2. The intra-particle diffusion model suggests that the process was diffusion controlled. The thermodynamic data indicates that the adsorption reaction is spontaneous with an increase in Gibbs free energy (∆G>0) and purely physisorption and exothermal in nature (∆H = -19.837 kJ/mol).
Keywords
Adsorption; Kinetics; Calcination; Physisorption; Pollution.
Introduction
The contamination of water by heavy metals have resulted to serious environmental pollution in the local community, leading to the development of studies aimed at their reduction or elimination by physical, chemical, thermal, biological or mixed means. The most common methods used to remove heavy metals from waste water are ion-exchange, reverse osmosis and chemical precipitation, but all these methods are too expensive. However, more attention has recently been paid to the sorption onto suitable materials such as activated carbon, clay and those making use of less expensive natural biomass [1].
It has been shown that the selectivity and efficiency in the removal of pollutants such as heavy metals from effluents by an adsorption process are strongly dependent upon the physical properties and chemical composition of adsorbents [2-3]. Kaolinite which is a doubled layered clay mineral with the chemical formula Al2O3·SiO2·2H2O (though consisting of other elements such as Sodium, Magnesium, Iron etc.), undergo phase transformation on heating to a certain high temperature (calcinations). It forms a spongy structure with pores upon calcination and so can be used as an adsorbent for heavy metals [4-7].
Among heavy metals, nickel is one of the most widely used in western society for the manufacture of stainless steel, super alloys, metal alloys, coins, batteries, etc. Direct exposure to nickel causes dermatitis. Some nickel compounds, such as nickel carbonyl, are cancinogenic and easily absorbed by the skin. The exposure to this compound, at an atmospheric concentration of 30ppm for half an hour is lethal [1,8,9].
There have been reports of water pollution by nickel metal in several communities [10] and several locally available materials such as Kankara kaolinite have been speculated to be effective in mopping up the pollutant from the environment, but its use as adsorbent has not yet been studied.
The aim of this research paper was to determine the adsorption kinetics of heat treated kaolinite so as to better understand and optimize its role as adsorbent for nickel ion in solution. Kankara kaolin is cheap and readily available and this knowledge will be a contribution to the global fight against pollution.
Material and Method
A certain amount of the grinded kaolinite clay was poured into a fabricated crucible and was then charged into an electric furnace. It was then heated to a temperature of 500°C for 24 hours. The surface area and volume of pores and micro-pores were obtained by N2 physisorption (BET method) at 77K. These analyses were performed using Micromeritis Tristar 3000 surface area analyzer device using the BET method. The analyses mentioned were carried out for raw and calcined Kaolinite clay 100mg of nickel salt (Ni(NO2)3·6H2O) was weighed and dissolved in 100ml of de-ionized water to obtain a standard solution of 1g/L. Thereafter, other standards were prepared from the standard of 1g/L by adding a calculated volume of the salt solution to a calculated volume of water using the dilution formula. The calibration curve was plotted and the calibration equation used to obtain the equilibrium and final concentration of the standard solutions used in adsorption. The standards were prepared using the equation of dilution as shown below:
|
C1V1 = C2V2 |
(1) |
where: C1=concentration of salt solution required, V1=corresponding volume at concentration C1 which may be guessed, C2=concentration of salt solution from which various standards were made, V2=volume of salt solution at concentration C2 which is calculated.
The adsorption experiments were carried out using an aqueous solution with (Ni (NO2)3·6H2O) of various known concentration in a beaker at controlled temperature under a specified stirring speed. Different masses of crushed kaolinite clay was weighed and a specified mass was of the clay was added to a known concentrate of nickel solution in a beaker after its pH have been controlled.
The beaker and its content was then placed on an electrical heating plate and heated at a given temperature with stirring at a specified stirring speed for a given time. The beaker and its content were removed and the content filtered. The absorbance of the resulting filtrate is then determined using an UV absorption photo spectrometer at a wavelength of 340nm. The experiments were conducted for each of the standards prepared and the kinetic models of adsorption were studied.
Adsorption Kinetics. The kinetic equations for adsorption process are:
|
ln(qe-q)=lnqe-k1t |
(2) |
|
|
(3) |
|
q=ki·t0.5+C |
(4) |
where: qe,qe2 are the equilibrium amount adsorbed; q is the amount adsorbed per time t; ki, k1, k2 are equilibrium constants; C is the intercept in equation (4).
Adsorption Isotherms (Effect of Temperature). The parameters of these models, expressed by equations (5) and (6) were associated with the process temperature. Using the adsorption isotherms, the maximum adsorbed capacity is observed to increase as the process temperature rises, which means that an increase in energy favours the adsorption on the clay surface. This behaviour indicates an enthalpy variation during positive adsorption or endothermic process. However, this behaviour is inverted in Henry’s infinite dilution region, that is, at very low concentration.
|
|
(5) |
|
lnqe=lnkf+(lnCe)/n |
(6) |
where: qm is the maximum amount absorbed; Ce is the equilibrium concentration; b is Henry’s constant, kf is the equilibrium constant.
Results and Discussion
From table 1, an increase in the surface area (7.9541m2/g to 9.1217m2/g) as well as pore volume and size is observed when kaolinite clay is calcined. The replacement of Na+ cations with Ni2+ cations led to an increase in the interplanar distance of the clay, which may account for the increase in the surface area of the clay following the nickel adsorption process [2].
Table 1. Sample surface area by BET method
|
Kaolinite clay |
BET surface area (m2/g) |
Pore volume (cm3/g) |
Pore size (nm) |
|
Raw |
7.9541 |
0.05410 |
24.56000 |
|
Calcined |
9.1217 |
0.05922 |
25.57349 |
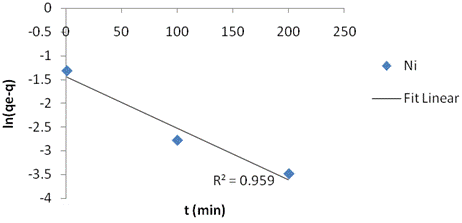
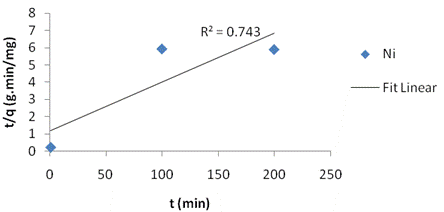
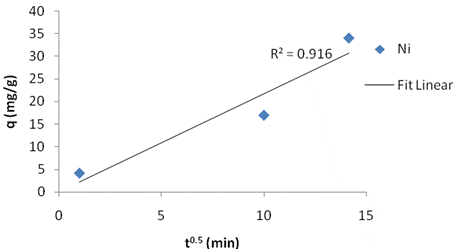
The adsorption kinetics was adjusted with the pseudo first order (figure 1a), the second order kinetic model (figure 1b) and the intraparticle diffusion model (figure 1c). The linear correlation coefficients and pseudo first order constant (k1), second order constant (k2) and intraparticle diffusion constant (ki) as well as the equilibrium capacity were obtained. From the slope and intercept, the calculated value of k1=0.025, k2=0.00065 and ki=2.089 g/(mg·min). Table 2 present the Adsorption kinetics for kaolinite Clay.
Table 2. Adsorption kinetics for kaolinite Clay
|
Cf (mg/L) |
Ce (mg/L) |
t (min) |
q (mg/g) |
qe (mg/g) |
ln(qe-q) |
t/q |
t0.5 |
|
6.94 |
170.06 |
200 |
34.012 |
1.388 |
-3.485 |
5.880 |
14.142 |
|
7.89 |
169.11 |
100 |
16.911 |
0.789 |
-2.780 |
5.913 |
10 |
|
3.62 |
163.38 |
1 |
4.085 |
0.341 |
-1.320 |
0.245 |
1 |
|
a) |
|
b) |
|
c) |
|
Figure 1. Fit for (a)pseudo-first order kinetic model; (b ) second-order kinetic model; (c)intraparticle kinetic model to Ni(II) adsorption on calcined kaolinite clay |
The intra-particle diffusion model was not suitable for experimental data when compared with the second order kinetic model, indicating that the diffusion of Ni species into the pores is the prevailing factor controlling the process mechanism. The first order kinetics model showed excellent linearity (R2=0.959) and tends to represent more satisfactorily the mechanism of interactions involved during nickel adsorption into the kaolinite clay pores.
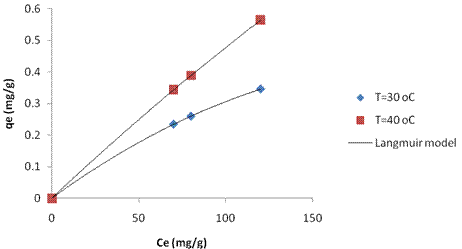
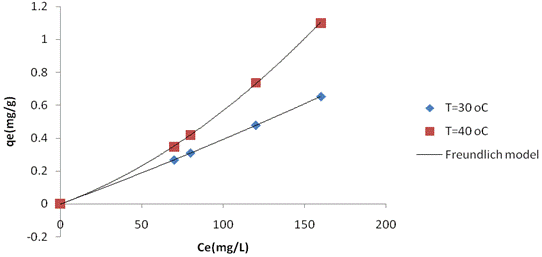
Adsorption Isotherms were obtained, which associate equilibrium concentrations in the solid and liquid phases at a given process temperature. Figure 2 and 3 shows the adsorption Isotherms for 1.0 g clay/100mL adsobate solution, adjusted by the Langmuir and Freundlich models, at initial nickel concentration ranging from 61.75 to 177mg/L. min). In table 3 is presented the Langmuir parameters, and in table 4 the Freundlich parameters.
Table 3. Langmuir parameters
|
Ce (mg/L) |
At T = 30°C |
At T = 40°C |
||||
|
qe (mg/g) |
qm(mg/g) |
B |
qe (mg/g) |
qm(mg/g) |
b |
|
|
70 |
0.235 |
1 |
0.0044 |
0.344 |
5.680 |
0.00092 |
|
80 |
0.260 |
0.389 |
||||
|
120 |
0.346 |
0.565 |
||||
|
160 |
0.876 |
0.729 |
||||
|
|
|
Figure 2. Adsorption isotherms for calcined Kaolinite Clay adjusted to the model of Langmuir |
Table 4. Freundlich parameters
|
Ce (mg/L) |
At T=30°C |
At T=40°C |
||||
|
qe(mg/g) |
kf |
n |
qe(mg/g) |
kf |
N |
|
|
70 |
0.267 |
0.0027 |
0.956 |
0.347 |
0.0009 |
0.717 |
|
80 |
0.309 |
0.418 |
||||
|
120 |
0.478 |
0.736 |
||||
|
160 |
0.652 |
1.100 |
||||
|
|
|
Figure 3. Adsorption isotherms for calcined Kaolinite Clay adjusted to the model of Freundlich |
It is also seen in figure 2 and Table 3 that the Langmuir model was the one that best fit the experimental adsorption isotherm data for two different process temperatures. The Langmuir isotherm is specific for monolayer adsorption, which was the case in this experiment while the Freundlich model is better applied to adsorption of heterogeneous sites on the surface of a solid, with a mechanism that has not been established yet. The Langmuir equilibrium adsorbate - adsorbent clay (solid phase) + Ni (II) (aqueous phase) = clay - Ni (II) moves. Higher values indicate that the equilibrium moves to the right side, with the resulting formation of the adsorbate - adsorbent complex [11].
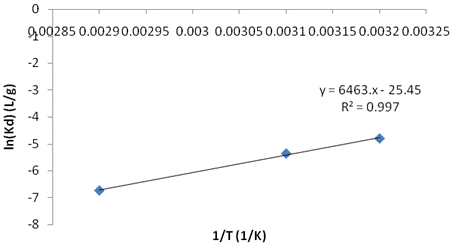
Adsorption Thermodynamics. The thermodynamic parameters ∆H, ∆S and ∆G presented in Table 5 were obtained from figure 4. The negative ∆H indicates that the process is exothermal, conferring the adsorption theory. The magnitude of the enthalpy variation achieved (-19.837 kJ/mol) is in the range of physisorption process, with weak vander waals bonds between the ions and the mineral clay. A decrease in entropy during adsorption helps the stabilization of the complex formed between the metal and the clay (∆S<0).
Table 5. Thermodynamic parameters of adsorption
|
T(°C) |
T(K) |
ΔG(J/mol) |
Kd(L/g) |
lnKd |
1/T |
ΔH(kJ/mol) |
ΔS(J/(Kmol) |
|
30 |
303.15 |
15.526 |
0.0021 |
-6.160 |
0.0033 |
-19.837 |
-0.110 |
|
40 |
313.15 |
12.507 |
0.0082 |
-4.804 |
0.0032 |
||
|
50 |
323.15 |
14.401 |
0.0047 |
-5.360 |
0.0031 |
||
|
75 |
348.15 |
19.468 |
0.0012 |
6.725 |
0.0029 |
||
|
At Co=177mg/L; R=8.314×10-3 kJ/mol/K |
|||||||
|
|
|
Figure 4. Langmuir parameter as a function of temperature |
The clay-Ni interactions occurred spontaneously and were not accompanied by a decrease in Gibbs free energy (∆G<0). Negative ∆S values suggest a decrease in randomness at the solid solution interface during nickel adsorption on Kaolinite clay. However, the degree of spontaneity was found to increase as the process temperature rose, ranging from 15.526J/mol to 19.468J/mol for the temperature range between 30 and 75°C [12].
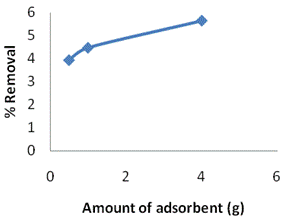
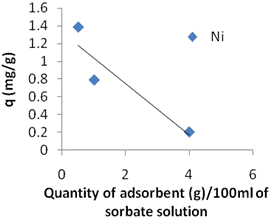
Effect of Adsorbent Amount. In figure 5a and Table 6, an increase in the removal percentage is noted as the amount of clay used increases at a constant concentration of 177mg/L. That is expected because at a fixed concentration of sorbate the increase in the adsorbent amount provides a larger surface area or adsorptive sites. However, according to figure 5(b) and Table 6 the adsorbed amount per unit mass of adsorbent (qe) declines. The decrease in adsorbed amount per unit mass of adsorbent is a generally observed behaviour.
|
a) in Ni(ii) removal |
b) at equilibrium |
|
Figure 5. Effect of the adsorbent amount on adsorption capacity |
|
Table 6. Effect of the adsorbed amount of Ni(II) at equilibrium on Kaolinite clay
|
Madsorbent (g) |
Ceq (mg/l) |
q (mg/g) |
% Removal |
|
0.5 |
170.06 |
1.388 |
3.92 |
|
1 |
169.11 |
0.789 |
4.46 |
|
4 |
169.01 |
0.200 |
5.64 |
|
At Co=177 mg/L |
|||
This may be attributed to two reasons:
1. A large adsorbent amount effectively reduces the un-saturation of the adsorption sites and correspondingly, the number of such sites per unit mass comes down resulting in comparatively less adsorption at higher adsorbent amount.
2. Higher adsorbent amount creates particle aggregation, resulting in a decrease in the total surface area and an increase in diffusion path length both of which contribute to decrease in amount adsorbed per unit mass [13,14].
Conclusion
The kinetic model of adsorption of nickel ion on Kankara kaolinite which is diffusion controlled can best be represented by the pseudo first order showing an excellent linearity of (0.959) and the thermodynamic parameter shows the process is physisorption and of exothermic nature.
Acknowledgements
This work was made
possible by part funding from the Petroleum Technology Development Fund (PTDF)
References
1. Jorge C., Kaolinite Properties, Structure and Influence of Metal Retention on pH. Faculty of Engineering and Applied Science, Memorial University Of Newfoundland, Canada, 2003, 4, p.13-22.
2. Vieira M.G.A., Almeida Neto A.F., Gimenes M. L., da Silva M.G.C., Sorption Kinetics and Equilibrium for the Removal Of Nickel Ions from Aqueous Phase on Calcined Bofe Bentonite Clay, Journal of Hazardous Materials, 2010, 177(1-3), p. 362-371.
3. Chen W.J., Hsiao L.C., Chen K.Y., Metal Desorption from Copper (Ii)/Nickel (Ii) Spiked Kaolin as a Soil Component Using Plant-Derived Saponin Biosurfactant, Process Biochem, 2008, 5, p. 488-498.
4. Grim R.E., Clay Mineralogy,
5. Kaolinite, Available at:
http://en.wikipedia.org/wiki/Kaolinite (accessed
6. Edomwonyi-Otu
L.C., Aderemi B.O., Ahmed A.S., Coville N.J., Maaza M., Influence of Thermal
Treatment on Kankara Kaolinite,
Opticon1826, 2013. 0(15):5, Available at: http://dx.doi.org/10.5334/opt.bc (accesed
7. Frost R.L., Hydroxy Deformation in Kaolin, Clays and Clay Minerals, 1998, 1, p. 280-289.
8. Seet R.C.S., Johan A., Teo C.E.S., Gan S.L., Chest K.H.L., Inhalational Carbonyl Poisoning in Waste Processing Workers, ProQuest medical Library, 2005, 424, p.6-9.
9. Zari H., Ghasem R.B., Yousef G., Sonication Enhanced Removal of Nickel and Cobalt Ions from Polluted Water Using an Iron Based Sorbent, Journal of Chemistry, 2013 (2013), Article ID 786954, Available at: http://dx.doi.org/10.1155/2013/786954 (accesed 30/03/2013)
10. Galadima A., Garba Z.N., Leke L., Almustapha M.N., Adam I.K., Domestic Water Pollution among Local Communities in Nigeria-Causes and Consequences, European Journal of Scientific Research. 2011, 52(4), p.592-603.
11. Igwe J.C., Abia A.A., Adsorption
isotherm studies of Cd (II), Pb
(II) and Zn (II) ions bioremediation from aqueous solution using unmodified and
12. Abbas S.T., Isotherm, Kinetic and Thermodynamic of Adsorption of Heavy Metal Ions onto Local Activated Carbon, Aquatic Science and Technology 2013, 1(2) p.53-77.
13. Shukla A., Zhang Y.H., Dubey P., Margrave J.L., Shukla S.S., The Role of Sawdust in the Removal of Unwanted Materials from Water, J. Hazard Mater, 2002, 95, p.137-152.
14. Osu C.I., Odoemelam S.A., Studies on Adsorbent Dosage, Particle Sizes and Ph Constraints on Biosorption of Pb(II) and Cd(II) Ions from Aqueous Solution Using Modified and Unmodified Crasstrotrea Gasar (Bivalve) Biomass, International Archive of Applied Sciences and Technology, 2010, 1(1), p. 62-68.