Development of Bone Ash and Bone Particulate Reinforced Polyester Composites for Biomedical Applications
Isiaka Oluwole OLADELE1,2*
1 Department Metallurgical and Materials Engineering, Federal University of Technology, Akure, Nigeria
2 African Materials Science and Engineering Network (AMSEN): A Carnegie-IAS (RISE) Network
E-mail: wolesuccess2000@yahoo.com *
* Corresponding author: Phone: +2348034677039
Abstract
This work was carried out to comparatively study the reinforcement efficiency of bone ash and bone particles on the mechanical properties of polyester matrix composites in order to investigate the suitability of the materials as biomaterial. Cow bone were procured from an abattoir and sun dried for 4 weeks after which it was crushed with a sledge hammer. A portion was burnt to ashes while others were further pulverized with laboratory ball mill before the two grades were sieved using 75 μm sieve size. Bone ash and bone particle reinforced tensile and flexural composite samples were developed from pre-determined proportions of 2, 4, 6, and 8 %. The samples after curing were striped from the moulds and were allowed to further cure for 3 weeks before tensile and flexural tests were performed on them. The tensile test result showed that, 8 wt % bone particle reinforced polyester composites has higher tensile properties except for modulus of elasticity where 8 wt % bone ash particle reinforced composites has higher value while for flexural test, bone ash particle reinforced composites demonstrate the best flexural properties. The results show that these materials are structurally compatible and being developed from animal fibre based particle, it will also aid the compatibility with the surface conditions as biomaterial.
Keywords
Bone; Polyester; Composites; Mechanical Properties; Biomedical.
Introduction
Biomaterials are materials of natural or man-made origin that are used to direct, supplement, or replace the functions of living tissues of the human body [1]. The use of biomaterials dates far back into ancient civilizations. Artificial eyes, ears, teeth, and noses were found on Egyptian mummies [2]. Chinese and Indians used waxes, glues, and tissues in reconstructing missing or defective parts of the body. Over the centuries, advancements in synthetic materials, surgical techniques, and sterilization methods have permitted the use of biomaterials in many ways [3]. Medical practice today utilizes a large number of devices and implants. Biomaterials in the form of implants (sutures, bone plates, joint replacements, ligaments, vascular grafts, heart valves, intraocular lenses, dental implants etc.) and medical devices (pacemakers, biosensors, artificial hearts, blood tubes etc.) are widely used to replace and/or restore the function of traumatized or degenerated tissues or organs, to assist in healing, to improve function, to correct abnormalities and thus improve the quality of life of the patients. Biomaterials are expected to perform in our body’s internal environment, which is very aggressive. During daily activities bones are subjected to a stress of approximately 4 MPa whereas the tendons and ligaments experience peak stresses in the range of 40-80 MPa. The mean load on the hip joint is up to 3 times body weight (3000 N) and peak load during jumping can be as high as 10 times body weight. More importantly, these stresses are repetitive and fluctuating depending on the activities such as standing, sitting, jogging, stretching, and climbing [4]. In a year, the stress cycles of finger joint motion or hip joint motion was estimated to be as high as 106 cycles and for a typical heart 0.5·107-4·107 cycles. This information roughly indicates the acute and instantaneous biological environment in which the biomaterials need to survive. Needless to say, the biological environment also depends on the patient’s condition and activities.
In the early days, all kind of natural materials such as wood, glue, rubber and tissues from living forms, and manufactured materials such as iron, gold, zinc and glass were used as biomaterials based on trial and error. The host responses to these materials were extremely varied. While some materials were tolerated by the body others were not. Also, under certain conditions, some materials were tolerated by the body, whereas the same materials were rejected in another situation. Over the 40 years, considerable progress has been made in understanding the interactions between the tissues and the materials. It has been acknowledged that there are profound differences between non-living (avital) and living (vital) materials. Researchers have coined the words biomaterials and biocompatibility to indicate the biological performance of materials. Materials that are biocompatible are called biomaterials, and the biocompatibility is a descriptive term which indicates the ability of a material to perform with an appropriate host response in a specific application. This definition has been further extended by Wintermantel and Mayer [5], and they distinguished between surface and structural compatibility of an implant [6]. Surface compatibility means the chemical, biological, and physical suitability of an implant surface to the host tissues while structural compatibility is the optimal adaptation to the mechanical behaviour of the host tissues.
Bio-composites from renewable resources gained much importance universally, because of their biodegradable nature. Bio-composites are most suitable materials profound in nature for their use in various fields due to their eco-friendly advantages. Bio-composites are manufactured using biopolymer as binder and natural fibre as the reinforcement material. Now-a-days research in polymer science and technology is mainly focused on composites made from renewable resources [7].
Polymers from natural sources are particularly useful as biomaterials and in regenerative medicine, given their similarly to the extracellular matrix and other polymers in the human body. Polyester- and polyether-urethanes have been modified with hydroxypropyl cellulose aiming the change of their surface and bulk characteristics to confer them biomaterial qualities [8-10]. Respecting dynamic contact angle measurements, dynamic mechanical analyses accompanied by mechanical testing have been done. Platelet adhesion test has been carried out in vitro and the use of hydroxypropyl cellulose in the polyurethane matrix reduces the platelet adhesion and therefore recommends them as candidates for biocompatible materials. Polymeric composites prepared by mixing polyurethanes and natural polymers offer improved mechanical properties and biocompatibility for functional tissue replacements in vivo. The biological characteristics in contact with blood and tissues for long periods, in particular good antithrombogenic properties, recommend the use of extracellular matrix components such as collagen, elastin and glycosaminoglycans (GAG) for obtaining biomaterials [11-15]. The introduction of biodegradable polymers into a synthetic polymer matrix restricts the action of a fungal, microbial or enzymatic attack [16]. Such limitations appear even when the biodegradable component occurs as a continuous phase in the composite material. Segmented polyurethanes have gained considerable position as useful biomaterials for implants or biomedical devices [17-19].
Clinical experience clearly indicates that not all commonly used engineering materials are suitable for biomedical applications. The various materials used in biomedical applications may be grouped into; metal, ceramic, polymer and composites. Of these groups, polymer and polymer based composites have been highly utilized but have not been explored extensively.
The aim of this research was to utilized bone ash and bone particles from cow bone to reinforce polyester material in other to develop a composite that is suitable for biomedical applications. Cow bone ash and bone particles are biodegradable animal fibres. Being animal fibres, they are expected to have good surface compatibility in addition to the structural compatibility obtained as biomaterials.
Material and Method
The main materials that were used for this work are as follows: unsaturated polyester resin, cow bone, Methyl Ethyl Ketone Peroxide (MEKP), Cobalt 2% in solution, polyvinyl acetate and ethanol.
Material Preparation: the cow bone was procure from the abattoir and sundried for 4 weeks after which some were burnt into ash while the remaining ones were crushed with hammer and finally pulverized using laboratory ball mill. The particles from both processes were sieved with a sieve size of 75 µm.
Mould Production: tensile mould of gauge length 90x5x3mm of a dumb-bell shape and flexural mould of 150x50x3mm were used for the production of tensile and flexural samples respectively.
Production of Composites: to develop the composites, 1.5g each of catalyst and accelerator was added to 120g of the polyester resin while the bone ash and bone particulate was varied in a predetermined proportion of: 2, 4, 6, and 8 wt%. After proper stirring, the homogenous slurry is poured into the mould and allowed to cure at room temperature before it is removed. The cured sample is left for 3 weeks before the mechanical tests were carried out.
Mechanical Testing and Structural Characterization of Cast Samples: following the moulding of the composites, samples were prepared for tensile, flexural and hardness tests. Scanning Electron Microscope (SEM) was used to investigate the miscibility between the fibre and matrix at the fractured surfaces. These tests were carried out as follows:
a) determination of the tensile properties of the materials: in the present study, tensile tests were performed on INSTRON 1195 at a fixed Crosshead speed of 10 mm min-1. Samples were prepared according to ASTM D412 (ASTMD412 1983) and tensile strength of the standard and conditioned samples were calculated.
b) determination of the flexural property of the materials: flexural test was carried out by using Testometric Universal Testing Machine in accordance with ASTM D790. To carry out the test, the grip for the test was fixed on the machine and the sample that has been cut into the test piece dimension of 150mmx50mmx3mm, was hooked on the grip and the test commenced. As the specimen is stretched the computer generates the required data and graphs. The Flexural Test was performed at the speed of 100 mm/min.
c) determination of the hardness property of the materials: hardness test was carried out in accordance with ISO R 868, using shore D. The test was carried out by impressing the sample with the tip of the indenter for five seconds before taking the readings from the calibrated scale. Ten readings were taking for each sample and the average value was used.
Results and Discussion
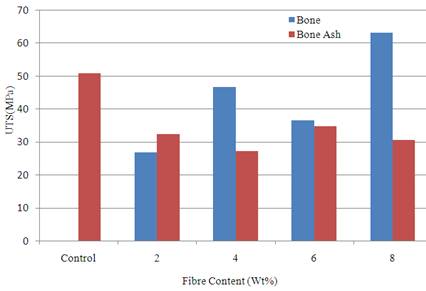
Figure 1 show the variation of ultimate tensile strength with the fibre content for the cow bone ash and cow bone particulate. It was observed from the result that sample reinforced with 8 wt % of particulate bone enhanced the tensile strength of the polyester matrix. The strength was 63.04 MPa compared to the unreinforced polyester matrix that has a value of 50.76 MPa.
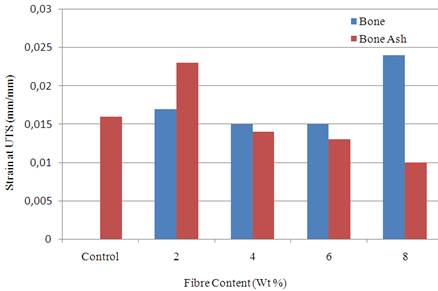
Figure 2 present the result of strain at ultimate tensile strength which is the parameter that measures the ability of the material to withstand plastic deformation without fracture. From the result, it was observed that sample reinforced with 8 wt% of particulate bone enhanced the strain at ultimate tensile strength of the polyester matrix. The strain was 0.024 mm/mm compared to the unreinforced polyester matrix that has a value of 0.016 mm/mm.
|
|
|
Figure 1. Variation of ultimate tensile strength with fibre content |
|
|
|
Figure 2. Variation of strain at ultimate tensile strength with fibre content |
The results from Figures 1-2 show that 8 wt % bone particle reinforcement gave the best tensile properties compared to other samples. The tensile strength value obtained from this sample fall within the range of values stated by [4]. Generally, in biomaterials, high strength of excess value is undesirable as this produces adverse bone remodelling and stress shielding, which over the long term leads to reduction in bone mass and implant loosening, especially in the proximal region. Fibre composites can be tailored to match the specific mechanical properties of the adjacent bone [20-21]. This is done in order to avoid stress accumulation and system damage.
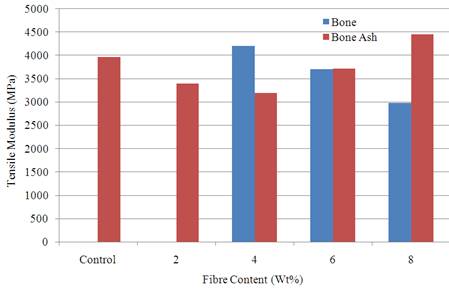
Variation of tensile modulus with fibre content is shown in Figure 3 where it was observed that 8 wt % bone ash reinforced polyester composite gave the best result. The value was 4450.49 MPa compared to unreinforced polyester matrix with a value of 3966.15 MPa. The tensile modulus is a parameter that measures the stiffness of the material. The result show that the tensile modulus increases as the fibre content increases from 4-8 wt % for bone ash reinforced samples while it decreases for bone particulate reinforced samples. 8 wt % bone particle reinforced composite has the least tensile modulus and as well stiffness. This is actually desirable for biomedical application as implants are not expected to be too stiff or rigid.
|
|
|
Figure 3. Variation of tensile modulus with fibre content |
|
|
|
Figure 4. Variation of flexural strength at peak with fibre content |
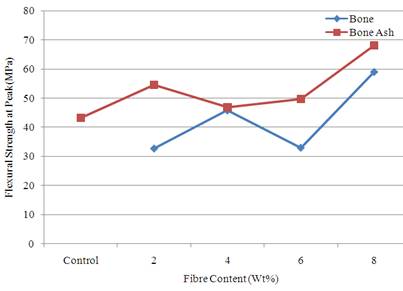
Figure 4 show the result of the flexural strength at peak for the various samples. From the result, it was observed that flexural strength at peak was better enhanced by the addition of bone ash. However, the best reinforcement was achieved when 8 wt % of bone ash and bone particle were added. The values were 68.24 and 59.14 MPa respectively while the value for the unreinforced polyester was 43.25 MPa. Flexural property is a parameter that measures the ability of the material to resist deformation under bending stress.
|
|
|
Figure 5. Variation of flexural modulus with fibre content |
|
|
|
Figure 6. Variation of hardness value with fibre content |
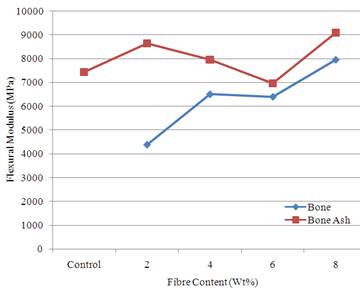
Figure 5 shows the variation of flexural modulus with fibre content. The result shows that bone ash reinforced samples possess better flexural modulus compared to bone particle reinforced samples. The highest value of 9103 MPa was obtained for sample reinforced with 8 wt % bone ash compared to unreinforced polyester matrix with a value of 7451.8 MPa.
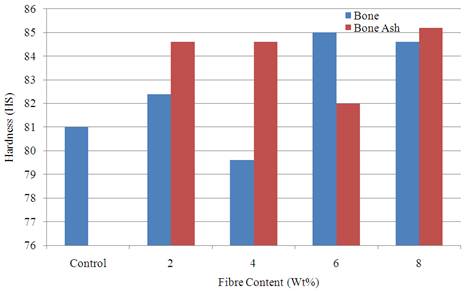
Hardness property is a measure of the resistance of the materials to surface indentation and wear. Figure 6 show the variation of this property with the samples from where it was noticed that the reinforcement leads to the enhancement of the hardness property in almost all the samples produced. Best result was obtained for 8 wt % bone ash reinforced sample with a value of 85.2 HS compared to the unreinforced polyester matrix with a value of 81 HS.
|
|
(a) Bone ash particulate reinforced polyester composites |
|
|
(b) Bone particulate reinforced polyester composites |
|
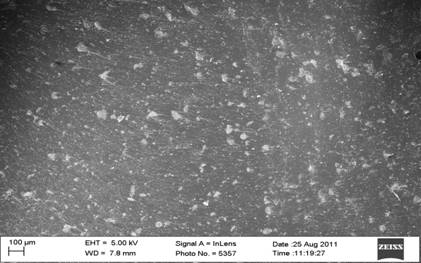
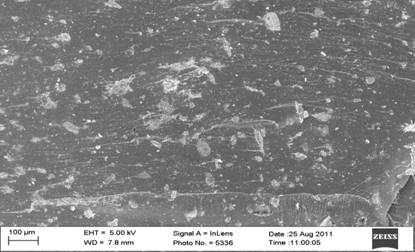
Figure 7. SEM micrograph of bone ash and bone particulate reinforced polyester composites |
|
Figure 7 depict the SEM micrographs of bone ash and bone particulates reinforced polyester composites (a-b). From the micrographs, it was observed that there is dispersal of the bone ash and bone particles (white particle) in the polyester (black surface). Mechanical tests results revealed that the particles were well dispersed in the polyester matrix as this was responsible for the better enhancement of the properties compared to the unreinforced polyester composites.
Conclusions
The work has been carried out to investigate the possibility of exploiting cow bone as reinforcement in polyester matrix for biomedical application. This was made for the purpose of biocompatibility which is an essential requirement in bio-composites that are to be used as implants in human body system. From the research, it was observed that cow bone being an animal fibre, can be used as reinforcement in polyester to develop composites for biomedical applications having met the structural conditions necessary. The following conclusions can also be drawn from the research:
a) Better results are obtained at high fibre content weight %. In all the properties investigated, 8 wt % gave the best results in both bone ash and bone particulate reinforced composite samples.
b) Both reinforcement materials (bone ash and bone particles) are potential materials for biomedical applications. However, bone particle reinforced composite developed from 8 wt % fibre content was the best. This was the situation because it was the best in tensile properties and also possesses good and favourable flexural and hardness properties. This sample (8 wt % bone particle reinforced) was able to meet the structural and surface conditions necessary for biomedical application.
c) The use of cow bone which is a natural animal waste is an economical and environmentally friendly driven research focus.
References
1. Black J., Biological Performance of Materials: fundamentals of Biocompatibility, Marcel Dekker, New York, 1992.
2. Black J., Hastings G.W., Handbook of Biomaterials Properties, Chapman and Hall, London, UK, 1998.
3. Park J.B., Biomaterials Science and Engineering, Plenum Press, New York, 1984.
4. Williams D.F., Consensus and definitions in Biomaterials, In: de Putter C., de Lange K., de Groot K., Lee A.J.C., editors, Advances in Biomaterials, Elsevier Science, Amsterdan, p. 11-16, 1988.
5. Wintermantel E., Mayer J., Anisotropic Biomaterials Strategies and Developments for Bone Implants, In: Wise D.L., Trantolo D.J., Altobelli D.E., Yaszemiski J.D., Gresser J.D., Schwartz E.R., editors, Encyclopedic Handbook of Biomaterials and Bioengineering, Part B-I, Marcel Dekker, New York, p. 3-42, 1995.
6. Wintermantel E., Ha S.W., Biocompactible Materials: Implant for Medicine, Berlin, Germany, Springer-Verlag, 1998.
7. Mohanty A.K., Misra M., Drazal L.T., SAMPE, Advanced Composite Technology for 21st Century Transportation, Midwest Advanced Materials and Processing Conference Proceedings, Dearborn, Michigan, p. 299, 2000.
8. Macocinschi D., Filip D., Vlad S., New Polyurethane Materials from Renewable Resources: Synthesis and Characterization, e-Polymers, 62, 2008.
9. Macocinschi D., Filip D., Butnaru M., Dimitriu C.D., Surface Characterization of Biopolyurethanes Based on Cellulose Derivatives, Journal of Materials Science:Materials in Medicine, 20(3), p. 775-783, 2009.
10. Macocinschi D., Filip D., Vlad S., Cristea M., Butnaru M., Segmented Biopolyurethanes for Medical Applications, Journal of Materials Science: Materials in Medicine, 20(8), p. 1659-1668, 2009.
11. Macocinschi D., Filip D., Vlad S., Surface and Mechanical Properties of Some New Biopolyurethane Composites, Polymer Composites, 31(11), p. 1956-1964, 2010.
12. Macocinschi D., Tanase C., Filip D., Vlad S., Oprea A., Study of the Relationship Between New Polyurethane Composites for Biomedical Applications and Fungal Contamination, Material Plastics, 47(3), p. 286-291, 2010.
13. Macocinschi D., Filip D., Vlad S., Cristea M., Musteata V.E., Ibanescu, S., Thermal, Dynamic Mechanical, and Dielectric Analyses of Some Polyurethane Biocomposites, Journal of Biomaterials Applications, 27(2), p. 119-129, 2011.
14. Moldovan L., Craciunescu O., Zarnescu O., Macocinschi D., Bojin D., Preparation and Characterization of New Biocompatibilized Polymeric Materials for Medical Use, Journal of Optoelectronics and Advanced Materials, 10(4), p. 942-947, 2008.
15. Musteata V.E., Filip D., Vlad S., Macocinschi D., Dielectric Relaxation of Polyurethane Biocomposites, Optoelectronics and Advanced Materials-Rapid Communications, 4(8), p. 1187-1192, 2010.
16. Raschip I.E., Vasile C., Macocinschi D., Compatibility and Biocompatibility Study of New HPC/PU Blends, Polymer International, 58(1), p. 4-16, 2009.
17. Hung H.S., Wu C.C., Chien S., Hsu S.H., The Behavior of Endothelial Cells on Polyurethane Nanocomposite and the Associated Signaling Pathways, Biomaterials, 30(8), p. 1502-1511, 2009.
18. Reddy T.T., Kano A., Maruyama A., Hadano M., Takahara A., Thermosensitive Transparent Semi-Interpenetrating Polymer Networks for Wound Dressing and Cell Adhesion Control, Biomacromolecules, 9(4), p. 1313-1321, 2008.
19. Wu Z.Q., Chen H., Huang H., Zhao T., Liu X., Li D., Yu Q. A., Facile Approach to Modify Polyurethane Surfaces for Biomaterial Applications, Macromolecular Bioscience, 9(12), p. 1165-1168, 2009.
20. Katz J., Orthopedic applications, in Biomaterials Science, Ratner B.D., (ed.), San Diego, Academic Press, p. 335-346, 1966.
21. Yildiz H., Ha S.K., Chang, F.K., Composite hip prosthesis design: Analysis, Journal of Biomedical Materials Research, 1998, 39(2), p. 92-101.