Preliminary Toxicological Evaluation of some Biochemical Parameters and Lipid Profile in Thevetia Neriifolia Seed Oil Supplemented Diet in Albino Rats
Sarah Onyenibe NWOZO 1, Ibironke Adetolu AJAYI2, and Bridget Ebehiremen IORLIAM 1*
1 Nutrition and Industrial Biochemistry Unit, Department of Biochemistry, College of Medicine, University of Ibadan, Ibadan, Nigeria.
2 Industrial Chemistry Unit., Department of Chemistry, University of Ibadan, Ibadan, Nigeria.
E-mail: sonwozo@yahoo.com; frajayi@yahoo.com ; brisuccess@yahoo.com *
* Corresponding author: Phone: +2348023658268
Abstract
Thevetia Neriifolia was analyzed for fatty acid composition by using Gas Chromatography (GC) and Gas Chromatography-Mass Spectrophotometer (GC-MS). The oil consist of 97.583% fatty acid of which the most abundant is the monounsaturated (C18H34O2) Oleic acid (52%), and the others are saturated fatty acids (C18H36O2) Stearic acid (25%) and the other Palmitic acid (C16H32O2). The physicochemical studies of the seed oil showed: acid value of 0.515 ± 0.27 mg KOH/g; 117.125±2.38 saponification value and 74.145 ± 0.784 iodine value. Growth performance in albino rats following a feeding period of 6 weeks, using 5%, 10% and 15% of the seed oil compounded feed showed a significant decrease in body weight gain and feed intake in 10% and 15% oil-based feed group relative to control. Enzymatic antioxidant, biomarkers of kidney/liver toxicity and lipid profile of visceral organs were evaluated.
Keywords
Chemical composition; Characteristics; Feed formulation; Growth performance; Toxicological evaluations.
Introduction
In the course of extensive study in search of new and potentially nutritional and industrially useful oils, we found out that several seed oils of several members of the Compositae family were shown to possess unusual properties such as a high yield of oil which contained trans conjugated diene and dienoic acid [1-6]. The relatively high proportion of the oils, suggested that they might have considerable industrial utility. Feeding experiments [7-8] and thermal studies [9] have shown that the oil has a very good replacement value for orthodox domestic vegetable oils. There is relatively minimal data on the toxicity of this seed oil; hence we carried out a comprehensive assay of the fatty acid composition and animal model nutritional study to evaluate the suitability of oil for consumption and possible toxicity. Thevetia Neriifolia plant is a dicotyledonous which belongs to Aponaceae family. It is an evergreen Compositae shrub with milky sap. It is native to West Indies, Mexico and Brazil. The common names are yellow oleader (nerium), gum bush, bush milk, exile tree in India, Cabalonga in Puerto Rico, Olomi ojo by Yorubas in Nigeria. The shrub reaches a height of about 3 to 3.9 meters. The plant is perennial; the leaves are linear, narrow sword like and green. The fruit when unripe is hard and green but gradually turns black as it ripens. The fruits have varying masses (2-6.1g) which are dispersed by man and propagated by seed or stem. The seed contain about 60-64% oil on dry matter basis. The plant produces a highly poisonous white latex sap [10]. Thevetia Neriifolia seed contains toxic compounds which are mostly cardiac glycosides and their free aglycones such as thevetic, digitoxigenin etc. [11-14] and has been shown to be toxic. The defatted seed cake however has a high level of toxicity [15-19] and it is most likely that the attention given to toxins has distracted interest from proper research of the oil and protein that would have promoted its industrial and domestic potentials.
In spite of the toxicity of the plant, it has been found useful in traditional medicine. The latex is used as an analgesic for toothache and the wood is used as axe handle. The seed consist of 60-65% oil and 35-40% protein [20]. The physical properties of the oil particularly the saponifiable and unsaponifiable matters shows that it can be used by commercial soap making industries.
It is interesting to note that, the cultivation of Thevetia Neriifolia shrub is relatively easy and the yield is very good. The shrub or tree can grow outside in warmer climates but in frost prone areas best brought back inside for winter and it tolerates most kinds of soil as long as they are well drained and is situated in full sun in a sheltered area. Useful as a landscaping plant in warmer climates as it does not need much maintenance.
It appears from literature search that, data on toxicity of Thevetia Neriifolia seed oil (TNO) is minimal; also the nutritional potential of TNO has not been evaluated.
The aim of this research was to evaluate the chemical characteristics and probable suitability of TNO as supplement in animal feed production.
Material and Method
Plant Identification and Preparation
The mature seeds were harvested from shrubs in University of Ibadan, Ibadan and identified in the Herbarium of the Department of Botany, University of Ibadan, Ibadan, Nigeria. A voucher specimen was deposited at the Departmental Herbarium. The fleshy fruits were dried and the soft portion removed to obtain the hard light brown seeds. The air dried seeds were crushed carefully to obtain the soft oily creamy pulp encased in dark brown seed coat. These were coarsely powdered using the dry cup of domestic Kenwood blender and subjected to Soxhlet extraction using n-Hexane for 6 hours in the Laboratory to obtain the oil.
Physicochemical Properties
The GC of the oil (as their methyl ester) was taken and the GC-MS was recorded on JEOL MS Route. Column and the flow rate of the carrier gas (helium) was 1-2 mls/min and injection temperature 250°C. Peaks were identified using authentic standards from computer literature search using fragmentation pattern. The percentage of each fatty acid component was calculated as the peak area percent of the total of all fatty acids. The various physicochemical properties such as acid value, saponification value and iodine value were determined as described by A.O.A.C [21].
Feed Components and Formulation
Soybean oil marked as Grand vegetable oil, a product of Grand Cereals and Oil mills Limited, Bukuru, Jos, Nigeria, yellow maize (Zea mays) grains and soy beans were purchased from Bodija Market, Ibadan, Nigeria. Vitamin mix was from BASF Aktiengesellschaft, Ludwigshafen, Germany. The composition of test feed per kg diet is as shown on table 1. Briefly, Corn starch was carbohydrate source, it was cleaned, sundried and milled. Soybean was first dehusked before milling and it was used as protein source. Components of the mineral mix was from Sigma-Aldrich Co. Ltd., Poole Dorset, UK The different components of the diet were thoroughly mixed, made into pellets for easy handling by animals and was thoroughly oven dried to prevent mould growth was stored in air tight bags at 4°C to prevent microbial contamination and auto-oxidation of the oil [22].
Table 1. Effect of milk bush (Thevetia Neriifolia) seed oil on body weight and feed-intake of rats fed with varying percentage of the oil
|
Treatment |
Initial body weight |
Final body weight % |
Weight gain |
Feed intake |
|
Control A |
92.33 ± 10.32 |
140 ± 25.15 |
44.67 ± 28.38 |
112.74 ± 10.88 |
|
Veg. oil B |
81.67 ± 4.08 |
161 ± 27.02 |
79.33 ± 24.87 |
69.56 ± 16.72 |
|
5% TNO C |
86.67 ± 6.06 |
142 ± 25.15 |
55.33 ± 24.97 |
62.84 ± 18.83 |
|
10% TNO D |
87.50 ± 5.24 |
103 ± 16.43* |
15.50 ± 17.32 |
59.60 ± 19.86* |
|
15% TNO E |
85.83 ± 3.77 |
107 ± 10.95* |
21.17 ± 11.77 |
56.11 ± 16.03* |
|
The results are present as mean ±S.D of six rats in each group *P<0.05 is significant when compared with the control |
||||
Proximate Analysis of the Feeds
The 5%, 10%, 15% milk bush oil feed, 100% pure commercial oil and commercially purchased rat chow from Ladokun feeds were analyzed for ash and mineral content using muffle furnace at 550°C for 4h. Moisture content was determined by drying in the oven at 100°C until a constant weight was obtained (at least 24 h). Total dietary fiber was determined by an enzymatic gravimetric method [23]. Crude oil content was assayed by extraction with n-hexane in a Soxhlet extractor [24]. Nitrogen was determined by standard micro kjeldahl method 922 using a digestion apparatus. The crude protein content was thereafter calculated by multiplying nitrogen by a factor of 5.71, which takes into account the non-protein nature of part of the nitrogen and has been approved for calculating the crude protein content of soybeans [25-26].
Animal Used
Thirty male albino rats (Wistar strain) weighing between 70g and 80g were obtained from the animal house in the Department of physiology university of Ibadan. On arrival, the rats were transferred and allowed to acclimatized, been maintained on the standard normal diet with water ad libitum in the Biochemistry department animal house under normal room temperature before the commencement of the experiment.
Experimental Design
Animals were distributed randomly into five different groups of six animals each. The control group received diet (commercially available rat feed), group A compounded feed using Grand soybean oil purchased from Bodija market, Ibadan, group B compounded diet with 5 % of the milk bush seed oil, group C compounded diet with 10 % of the milk bush seed oil and group D compounded diet with 15 % of milk bush seed oil.
Sample Collection
Animals were sacrificed by cervical dislocation and blood was obtained using 2ml syringe by cardiac puncture into clean bottles and allowed to clot. These were spun at 3000 rpm for 10 minutes; the supernatant (serum) was removed and stored at 4°C. The liver and kidney were quickly removed, weighed, washed with 1.15% KCl, homogenized in 56 mM Tris-HCl buffer (pH 7.4) containing 1.15% potassium chloride and the homogenate was centrifuged at 10.000 rpm for 15 minutes to obtain post mitochondrial fraction (PMF) at 4°C.
Biochemical Analysis
The concentration of protein in the serum and PMF was determined using the method Lowry et al. [27] with BSA as standard. The extent of lipid peroxidation was determined by estimating the thiobarbituric acid reactive substances (TBARS) formed following the method of Varshney and Kale [28]. Microsomal catalase (CAT) activity was determined by using hydrogen peroxide, briefly, the reaction mixture contained phosphate buffer (0.01 M, pH 7.0), tissue homogenate and 2 M H2O2. The reaction was stopped by the addition of dichromate-acetic acid reagents (5% potassium dichromate and glacial acetic acid were mixed in a ratio of 1:3) [29]. Cholesterol was determined according to the method of Richmond [30] by absorbance measurement at 490 nm when cholesterol reacts with FeS04 in glacial acetic acid is treated with H2SO4. Triglycerides level was estimated after enzymatic hydrolysis with lipases by measuring quinoneimine formed from hydrogen peroxide, 4-aminophenazone and 4-chlorophenol under catalytic influence of peroxidase [31].
Urea estimation was done using kit supplies by Dialab production and Vertrieb vonchemisch Technischen. Urease hydrolysis urea to ammonia and carbondioxide and the ammonia reacts with alkaline hypochlorite and sodium salicylate to produce a colored complex which is measured spectrophotometrically at 546 nm. Enzyme parameters Aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) were assayed using Randox kits.
Statistical Analysis
Data was expressed as mean ±S.D of six determinations, except for the proximate analysis and were analyzed using one way analysis of variance (ANOVA) and complimented with student t-test. Values for p < 0.05 were considered to be statistically significant.
Results and Discussion
The yield calculated from the oil extraction was approximately 58.03% oil on dry matter basis. We obtained seed oil, faintly whitish in color, with no characteristic smell and semi-solid at room temperature from hexane extraction. The physicochemical studies of the seed oil showed: acid value of 0.515±0.27 mg KOH/g; 117.125±2.38 saponification value and 74.145±0.784 iodine value.
Data obtained from the change in body weight, change in weight of visceral organs and feed intake of animal on the compounded milk bush oil meal, commercially sold vegetable oil and regular rat chow are shown on tables 1 and 2. Percentage gain in body weight was highest in the commercially sold vegetable oil group (79.33±24.87); followed by the 5% TNO with 55.33% and almost 300% decrease in body weight was observed in 10% and 15% (Group D&E) seed oil supplemented groups. Interestingly the growth performance in commercially consumed vegetable oil was about 60% of the commercial rat chow.
Table 2. Effect of milk bush (Thevetia Neriifolia) seed oil on weight of organs of rats fed with varying percentage of the oil
|
Treatment |
Weight of liver |
Weight of kidney |
Weight of heart |
|
Group A |
3.00 ± 0.00 |
1.03 ± 0.10 |
0.51 ± 0.23 |
|
Group B |
2.73 ± 0.29* |
0.64 ± 0.14* |
0.25 ± 0.09 |
|
5 % seed oil C |
2.96 ± 0.90 |
0.78 ± 0.07* |
0.40 ± 0.25 |
|
10 % seed oil D |
2.96 ± 0.09 |
0.78 ± 0.10* |
0.34 ± 0.08 |
|
The results are present as mean ±S.D of six rats in each group. *P<0.05 is significant when compared with the control. |
|||
Feed intake was almost 50% lower the groups with TNO seed oil supplementation and was equally lower than that of vegetable oil as shown on table 3. There was a significant decrease in the weight of the visceral organs when compared with the control (table 1) The groups fed with compounded diets with TNO and Grand vegetable oil (Groups B, C, D&E) showed significant decrease in the weight of the kidney when compared with the control. Furthermore there was 11.07 % significant (p < 0.05) decrease in the body weight of rats fed with 15 % of the seed oil when compared with the control. We observed a significant (p<0.05) decrease in feed intake when control group was compared with 10% and 15% (Groups D&E) on bush milk oil (TNO).
Table 3. Proximate analysis of commercial rat chow (control) and test diet with vegetable oil
|
Treatment |
% Moisture |
%Fat |
% Crude fiber |
%Ash |
%Protein |
|
Control diet |
8.83 |
4.88 |
9.22 |
7.38 |
18.39 |
|
Test diet |
7.83 |
3.96 |
5.56 |
7.30 |
18.19 |
The result of serum ALT, AST, ALP, Urea and protein are shown on table 4. AST was significantly increased compared to control, similarly ALT was about 300% higher in the 15% TNO supplemented oil group elevated serum AST significantly (p<0.05) when control group was compared with rats fed with 15% (Group E) of TNO. A non-significant decrease in AST was obtained when control group was compared with rats treated with 5% and 10% of the T neriifolia seed oil (Groups C and D). The result for ALT showed that there was a significant increase in serum ALT activities when 5%, 10% and 15% were compared with the control. The significant rise in these marker enzymes portrays a slight degree of impairment to the liver. There was a significant increase in serum AST, ALT and ALP when control (Group A) was compared with the test animals on pure grand vegetable oil diet (Group B). Urea was significantly increased in the control rats compared to test animals on pure Soybean oil feed (Group B), whereas the urea values was significantly increased in the 10% and 15% bush milk oil (Groups D and E) when compared with control. There were no significant changes in serum protein concentration in the animals used in this study.
Table 4. Effect of milk bush (Thevetia Neriifolia) seed oil on serum AST, ALT, ALP, Urea and Protein rats fed with varying percentage of the oil
|
Group |
AST (U/I) |
ALT (U/I) |
Urea |
ALP (U/I) |
Protein |
|
Control A |
14.50±1.73* |
2.25±0.30* |
164.62±14.9* |
48.85±5.70* |
5.54±o.8 |
|
Veg. oil B |
20.25±10.72 |
6.25±6.13 |
234.12±23.44 |
53.79±21.27 |
4.75±1.34 |
|
5% seed oil C |
24.05±3.56 |
18.75±4.99* |
216.47±36.25 |
70.29±33.6* |
5.51±0.9 |
|
10% seed oil D |
41.75±14.54 |
17.5±4.43* |
252..89±33.05* |
101.73±16.54* |
7.08± 1.8 |
|
15% seed oil E |
53.54±17.10* |
20.25±6.24 |
263.97±5.60* |
119.13±17.70* |
5.75±1.25 |
|
The results are present as mean ±S.D of six rats in each group. *P<0.05 is significant when compared with the control. |
|||||
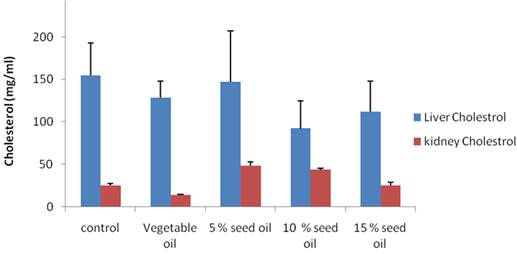
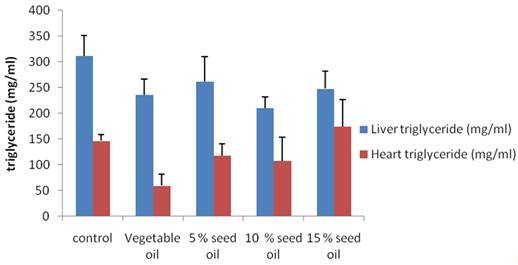
Liver cholesterol was lower in all groups of animals on test diet (compounded animal feed relative to control as shown in figure 1, however 10% TNO diet had the lowest (30%) liver cholesterol compared to the control group. Except for the group on 5% TNO, other animals on compounded feed had lower liver cholesterol compared to 100% pure vegetable oil group. On the other hand TNO supplementation elicited higher kidney cholesterol compared to only vegetable oil group and control group as depicted in figure 1. Data obtained for visceral organs triglyceride level is shown in figure 2. TNO supplementation produced higher levels of triglyceride in all the groups compared to only vegetable oil group. Only in the 5% and 10% TNO groups was the kidney triglyceride lower than control. In the liver all animals on both pure vegetable oil and TNO supplementation had lower triglyceride compared to control group.
|
|
|
Figure 1. Effect of bush milk oil based diet on liver and kidney cholesterol |
|
|
|
Figure 2. Effect of milk bush (Thevetia neriifolia) seed oil on liver and heart Triglyceride level |
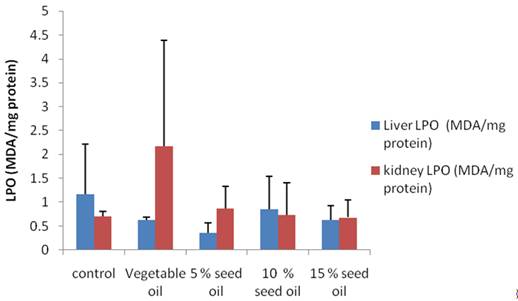
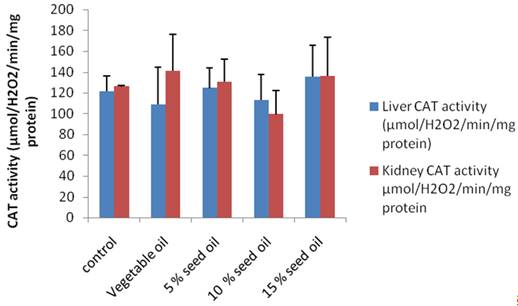
Data obtained for MDA levels, a measure of lipid peroxidation and catalase in both kidney and liver are shown in figure 3 and 4 respectively. Commercial vegetable oil elicited much higher LPO (over 100%) increase relative to both control and TNO supplemented groups in the kidney. Except for 5 % TNO feed group, other animals had lower Kidney MDA levels compared to control group. In the liver, TNO diet produced lower LPO compared to control and 5% TNO feed group produced the lowest MDA in that category as depicted in figure 3. Catalase activity was highest in both kidney and liver in animals on 15% TNO diet, while 5% TNO supplemented feed had catalase level which compared favourably with animals on control diet.
|
|
|
Figure 3. Effect of milk bush (Thevetia neriifolia) seed oil on liver and kidney MDA level |
|
|
|
Figure 4. Effect of Milk bush oil (Thervetia neriifolia) on liver and kidney Catalase activity |
The was percentage yield of milk bush seed oil we obtained in this study was slightly lower than 61-63% previously reported in the literature, but was not significantly different. GC and GC/MS analysis of the crude hexane extracted TNO showed the oil consists of mostly saturated and monounsaturated fatty acids of which the most abundant is Oleic acid, followed by Stearic acid and Palmitic acid and the oil was a whitish semi-solid at room temperature. The composition seemed comparable with consumable vegetable oils as palm oil (Eleaeis guineensis) and peanut oil (Arachis hypogeae). The physiochemical parameters we obtained were similar to earlier values reported by Ibiyemi et al. [20]. The saponification value compared favorably with most food oils and indicates that TNO oil might be useful for soap making, candle production and as chemical feedstock for lubricants.
There was a significant decrease in the weight of the visceral organs when compared with the control (table 1) and significant decrease in the feed intake when control group A was compared with 10% and 15% TNO compounded diet feed groups (D&E). Relating feeding experiment result to a distinguished single component is difficult due to presence of anti-nutritive factors in foods and feed intake should also be considered. Due to this, we have used the same food stuff for compounding the test feed except the oil where we supplemented 5, 10 and 15% TNO instead of 100% grand vegetable oil. The feed intake was greatly reduced in the groups on the compounded diets (Groups C, D&E) and this culminated in reduced body weight, except for the animals on pure commercially sold vegetable oil (table 2).
Previous study by Pahwar and Chatterjee [17] reported the toxic effects of yellow oleander seed kernels in the roof rat (Rattus rattus Linn) and the main signs of poisoning observed were hind limb paralysis, rolling of the body on the long axis, circular flailing of the tail, muscular twitch, tetanic convulsions, tremors, collapse and death. We did not observe any abnormal behavior or decreased psychomotor activity in animals fed the TNO oil based diet. However, bush milk oil elevated serum AST significantly (p<0.05) when control group was compared with rats fed with 15% (Group E) of TNO. A non-significant decrease in AST was obtained when control group was compared with rats treated with 5% and 10% of the T. neriifolia seed oil TNO (Groups C and D). Elevated serum ALT activities were obtained in 5%, 10% and 15% TNO (Groups C, D and E) were compared with the control (Group A). The significant rise in these marker enzymes portrays a slight degree of impairment to the liver. There was a significant increase in serum AST, ALT and ALP when control (Group A) was compared with the test animals on pure grand vegetable oil diet (Group B). Also, urea was significantly increased in the control rats compared to test animals on pure Soybean oil feed (Group B), whereas TNO significantly increased serum urea in the 10% and 15% bush milk oil (Groups D and E) when compared with control. There were no significant changes in serum protein concentration in the animals used in this study. Transaminases play an important role in protein and amino acid metabolism in hepatocytes and striated muscle cells. However, when cells are injured or necrosis occurs, these enzymes may leak or escape from these cells into the blood stream, where their present activities are consequently increased. Therefore, the determination of these transaminases in the serum is often used as one of the essential marker of liver damage. This is because they are cytoplasmic in location and are released into circulation after cellular damage [32]. The elevated values obtained for TNO supplemented groups for AST, ALT and ALP compared to Grand vegetable oil group (table 4) indicates that the commercially sold oil is not hepatotoxic as the T.nerilolia oil (TNO) and the hepatotoxicity increases with increase in the concentration of the oil in the formulated meal.
Liver cholesterol was significantly decreased in the animals on the 10% and 15% Bush milk diet (Groups D and E) relative to control and in the 5% (Group C) relative to the test diet group with pure soybean vegetable oil (Figure 1). The observed decrease in liver cholesterol might be due to the smaller size of the internal organs in animals on bush milk diet and the general reduction in bodyweight which we observed in this study. Cholesterol has been shown to reduce fatty acid oxidation, which in turn, increases the levels of hepatic and plasma triglyceride [33]. Liver triglyceride level was significantly reduced in the test diet (Soybean oil) group as well as in the 10% and 15% T neriifolia oil (Groups D and E) compared to control. Only in the 10% (Group D) bush milk oil group was the heart triglyceride level significantly reduced compared to animals on the test feed (Group B) with market sold vegetable oil. Lipid peroxidation (LPO) was only significantly reduced in the rats on 5% bush milk oil (Group C) compared to control in the liver, while the value obtained for kidney of animals on pure vegetable oil (Group B) increased by about 150%. There was a non-significant increase in catalase activity in the kidney of test animals relative to control while we observed a non-significant decrease in the liver of animal on soybean oil, 5% and 10% (Group C and D) bush milk seed oil based diet.
An overabundance of free radicals in the cell leads to uncontrolled chain reactions and lipid peroxidation, resulting in various pathological conditions that may include cardiovascular diseases, such as preeclampsia, atherosclerosis and cancer. An increase in the level of the LPO product (malondialdelyde) which is an index of the level of oxygen free radical, hence a decrease in LPO may lead to a reduction in the arterial wall cholesterol content. Lipid peroxidation contributes to the development of and the end product of this process, malondialdehyde (MDA), and 4-hydroxynonenal (HNE) can cause damage to proteins and DNA. Concentration of malondialdehyde (MDA), an index of lipid peroxidation was significantly reduced in the liver of rats on 5% (Group C) bush milk oil relative to control and it was lower than the commercially sold vegetable oil (Group B). Oxygen free radicals contribute to physiological endothelial cell injury, which appears to be an important component in the response to injury. Animals on 5% and 10% bush milk oil in (Groups C and D) had similar catalase enzyme activity as control animals. Histopathological photomicrograph (data not included) of the liver and the heart did not show any significant lesions, only in the 5% milk bush oil (Group C) did we have generalized fatty acid degeneration.
Observed increment in the activities of ALT, ALP and AST in the serum in the present study may be attributed to the leakage of these enzymes from the liver into the serum indicating the possible hepatotoxic effect of the test diet with TNO. We also had reduction in MDA in the liver of rats on 5% T. neriifolia oil (Group C) but higher values of TNO supplementation we obtained at higher liver MDA level. Although we did not observe any significant lesion in the visceral organs, TNO oil should be used with caution, especially when consumed over a long period. Also there was reduction in feed intake, which subsequently led to reduced body weight. The ingestion of Thevetia Neriifolia seed caused significant reductions in the rats' weights, increased BUN, SGOT and LDH significantly and histopathological studies showed inflammatory and degenerative changes in the liver and kidney [32]. Severe to moderate fatty metamorphosis, congestion, hepatocytolysis, nuclear degeneration, pyknosis, and necrosis were major changes observed in the liver of rats on ingestion of bush milk seed [32]. Atteh et al. [7-8] reported that the oil was safe for chicken feed. Our results have not shown that the consumption of the oil for prolonged period is safe in rats especially in the 10% and 15% (Groups D and E) bush milk oil compounded feed.
Conclusions
The result of the chemical composition of Thevetia Neriifolia seed oil (TNO) shows great similarity with conventional vegetable oil. This high oil rich seed grows easily without much attention and is grossly left to waste annually. Our study has shown the need to further purify the TNO for dietary purposes and the oil should be used with caution at above 5% replacement levels in rat feeds.
Acknowledgements
The author for correspondence is grateful to the Third World Academy of Science (TWAS) for postdoctoral fellowship in Centres of Excellence in the South utilized in International Centre for Chemical and Biological Sciences (ICBS), HEJ Research Institute of Chemistry, University of Karachi, Karachi, Pakistan where the GC and GC-MS were done.
References
1. Bochra L., Karima K., Taoufik B., Abdelaziz M., Brahim M., Essential oils and fatty acids composition of Tunisian, German and Egyptian caraway (Carum carvi L.), Seed Ecotypes: A Comparative Study, Industrial Crops and Products, 2013, 41, p. 312-318.
2. Wang M.L., Morris B., Tonnis B., Davis J., Pederson G.A., Assessment of Oil Content and Fatty Acid Composition Variability in Two Economically Important Hibiscus Species, J. Agric. Food Chem, 2012, 60, p. 6620-6626.
3. Zeb A., Triacylglycerols Composition, Oxidation and Oxidation Compounds in Camellia Oil using Liquid Chromatography-Mass spectrometry, Chemistry and Physics of Lipids, 2012, 165(5), p. 608-614.
4. Bettaieb I., Bourgou S., Sriti J., Msaada K., Limam F., Marzouk B., Essential oils and fatty acids composition of Tunisian and Indian cumin (Cuminum cyminum L.) seeds: A Comparative Study, J Sci Food Agric, 2011, 91, p. 2100-2107.
5. Mebrahtu T., Gebremariam T., Kidane A., Araia W., Performance of Vernonia galamensis as a potential and viable industrial oil plant in Eritrea: Yield and Oil Content, African Journal of Biotechnology, 2009, 8(4) p. 635-640.
6. Mollah J.U., Islam W., Toxicity of Thevetia peruviana (Pers) Schum. Extract to Adults of Callosobruchus maculatus F. (Coleoptera: Bruchidae), Journal of Agriculture & Rural Development, 2007, 5(1&2), p. 105-109.
7. Atteh J.O., Ibiyemi S.A., Onadepo F.A., Ugbona O.O., Replacement of Palm Oil by T. Peruviana Oil in Broiler Chick Diets, J. Agric Sci. Cambridge, 1990, 115, p. 114-143.
8. Atteh J.O., Ibiyemi S.A., Ojo A.O., Response of Broilers to Dietary Levels of T. Peruviana Cake, 1995, 125, p. 310-313.
9. Ibiyemi S.A., Bako S.S., Ojukuku G.O., Fadipe V.O., Thermal Stability of T.Peruviana Juss Seed Oil, J. Am. Oil Chem. Soc, 1995, 72(6), p. 745-747.
10. Ibiyemi S.A., Faloye T., Potassium, Nitrogen and Calcium Uptake by T. peruviana Seedlings as Affected by Various Nutrient Sources, Nig. J. Agronomy, 1998, 3(2), p. 68-73.
11. Sticher O., Theveside A., New Iridoid Glycoside from T.peruviana, Tet. Lett. 1970, 36, p. 3195-3196.
12. Sticher O., Thevesid a new iridoid-glycoside from Thevetia peruviana (Pers.) K. SCHUM (Thevetia Neriifolia Juss.) (Apocynaceae), Pharm Acta Helv, 1971, 46(3), p. 156-166.
13. Gupta O.P., Misra K.C., Arora R.B., Cardiotonic and Antiveratrinic Action of Thevetia neriifolia Juss Glycosides Compared with Ouabain and their Structure Activity Relationship, Indian J Exp Biol., 1974, 12(5), p. 399-401.
14. Abe F., Yamauchi T., Yahara S., Nohara T., Glycosides of 19-Formylthevetiogenin and 5 Alpha-Hevetiogenin from Thevetia neriifolia Phytochemistry, 1994, 37(5), p. 1429-1432.
15. Vohra M.M., Kohli J.D., De N.N., Pharmacological Studies on Ruvoside-Another New Digitaloid from Thevetia Neriifolia Juss, Arch Int Pharmacodyn Ther, 1961, 133, p. 265-274.
16. Verma B.B., The Cardiotonic and other Pharmacologic Actions of Thevetia neriifolia, Indian J Physiol Pharmacol, 1964, 8, p. 8-20.
17. Pahwa R., Chatterjee V.C., The Toxicity of Yellow Oleander (Thevetia neriifolia juss) Seed Kernels to Rats, Vet Hum Toxicology, 1990, 32(6) p. 561- 564.
18. Uber-Bucek E., Hamon M, Pham Huy C., Dadoun H., Determination of Thevetin B in Serum by Fluorescence Polarization Immunoassay, J Pharm Biomed Anal, 1992, 10(6), p. 413-419.
19. Bose T.K., Basu R.K., Biswas B., De J.N., Majumdar B.C., Datta S., Cardiovascular Effects of Yellow Oleander Ingestion, J Indian Med Association, 1999, 97(10), p. 407-410.
20. Ibiyemi S.A., Fadipe V.O., Akinremi O.O., Bako S.S., Variation in oil of Thvetia Peruvian Juss., J Appl. Sci Environ Management, 2002, 6(2), p. 61-65.
21. AOAC, Official methods of analysis (15th ed.), Association of Official Analytical Chemists, Arlington, V A, 1990.
22. Oladiji A.T., Abodunrin T.P., Yakubu M.T., Toxicological evaluation of Tetracarpidium conophorum nut oil based diet in rats, Food and Chem. Tox., 2010, 48, p. 898-902.
23. Pellett P.L., World Essential Amino Acid Supply with Special Attention to South East Asia. Food Nutritional Bulletin, 1996, 17, p. 204-234.
24. Petzke K.J., Ezeagu I.E., Prol J., Akinsoyinu A.O., Metges C.C., Amino acid composition, available lysine content and in-vitro protein digestibility of selected tropical crop seeds, Plant Foods Hum Nutr., 1997, 50, p. 151-162.
25. Baker K.M., Utterback P.L., Parsons C.M., Stein H.H., Nutritional value of soybean meal produced from conventional, high-protein, or low-oligosaccharide varieties of soybeans and fed to broiler chicks, Poult. Sci., 2011, 90, p. 390-395.
26. Garcia A.R., Batal A.B., Dale N.M., A Comparison of methods to determine Amino acid digestibility of feed ingredients for chickens, Poult. Sci., 2007, 86, p. 94-101.
27. Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J., Protein measurement with the Folin phenol reagent, J. Biol chem, 1951, 193, p. 265-275.
28. Varshney R., Kale R.K., Effects of Calmodulin Antagonists on Radiation induced Lipid Peroxidation in Microsomes, Int J Radiat Biol, 1990, 58, p. 733–743.
29. Claiborne A., Catalase activity, In: Handbook of Methods for Oxygen Radical Research, Edited by A. R. Greenwald, CRC Press, Florida, 1989, p. 234-242.
30. Richmond W., Preparation and Properties of Bacterial Cholesterol Oxidase from Nocardia Specie and its Application to the Enzymatic Assay of Total Cholesterol in Serum, Clinical Chemist, 1973, 19, p. 1350–1356.
31. Tietz N.W., Clinical Guide to Laboratory Test, Second Edition Published by Saunders (W.B.) Co Ltd, Philadelphia, USA, 1990. p. 554.
32. Salic R., Tredger J.M., William R., Drugs and the liver, Biopharmaceutical Drug Dispos, 1991, 12, p. 251-259.
33. Miettinen T.A., Tarpila S., Effects of Pectin on Serum Cholesterol, Feacal Bile Acids and Biliary Lipids in Normo- And Hyperlipidemic, Clinical Chemistry, Acta, 1997, 79, p. 471-477.