Corrosion Behaviour of Alpha Phase Aluminium Bronze Alloy in Selected Environments
Oluwayomi BALOGUN1, 2*, Joseph BORODE2, Kenneth ALANEME2, and Michael BODUNRIN2
1Prototype Engineering Development Institute, (PEDI) Ilesa,
2Metallurgical and Materials Engineering Department,
Emails: yomdass@yahoo.com; borode202@yahoo.co.uk; kkalanamek@yahoo.co.uk ; bodmas1000@yahoo.com.
* Corresponding author: Phone: +234-8036362432
Abstract
This research investigated the corrosion behaviour of aluminium (8 wt %) bronze alloys produced via sand casting in acidic, alkaline, and marine environments. The aluminium bronze was produced from aluminium (6063) alloy and copper scraps by sand casting according to European standard specification (UNS. C61400-CuAl8), after which they were cut into smaller sizes and immersed in the selected corrosive media for corrosion test investigation. H2SO4, NaCl, NaOH, and HCl of 0.1 M, 0.2 M, 0.3 M, 0.4 M, and 0.5 M were setup for 45 days for the corrosion study. Selective phase attack was observed in the alloy, although it was much more pronounced in HCl, to the point where entire grains fell out while it exhibit minimal corrosion resistance in marine and alkaline media respectively. Intense chloride attack on the protective film formed on the surface of the aluminium bronze was observed to be responsible for the greater corrosion susceptibility of the alloy in HCl environments. Comparative studies of aluminium bronze in selected environments indicated that no corrosion was observed and the alloys have a greater tendency to be applicable in marine, alkaline and sulphuric acid environments.
Keywords
Aluminium Bronze; Aluminium 6063; Copper Scraps; Sand Casting; Selective Phase Attack; Corrosion Test; Corrosive Media; Grains.
Introduction
Aluminium bronzes are copper based alloys with aluminum as the major alloying element usually in the range 5-11% compositionally in the alloy. Other alloying elements sometimes intentionally introduced are iron, nickel, manganese, silicon and tin depending on the intended application of the aluminium bronze [1]. They are available in both wrought and cast product forms, and offer good combinations of mechanical properties and corrosion resistance. Applications of aluminium bronzes include high pressure flange for sub-sea weapons ejection system, clutch components for shipboard winch, propellers, landing gear components on aircraft, wear rings for hydro-turbine, impellers shafts, pumps and valves, exchanger parts, non-sparking tools, tube sheets and lots more [2]. The aluminium bronzes in both cast and wrought forms have excellent corrosion resistance of all copper alloys enhanced by the protective film of aluminium oxide formed rapidly under normal oxidizing conditions. If damaged, this film is self-healing which means that the alloys can be used under service conditions where abrasion can be expected [3].They offer a combination of mechanical and chemical properties unmatched by any other alloy series and they show low rates of oxidation at high temperatures and excellent resistance to sulphuric acid, sulphur dioxide and other combustion products. Therefore, they are used for applications where their resistance to corrosion makes them preferable to other engineering materials [1].
These features often make aluminum bronzes the first choice and sometimes the only logical choice for demanding applications [4]. Aluminum bronzes are most valued for their high strength and corrosion resistance in a wide range of aggressive media. Increasing the aluminum content results in higher strength, which is attributable to a hard, body-centered-cubic phase, which enhances properties of castings as well as hot working in wrought alloys [5].Besides their strength, toughness, corrosion resistance in a wide range of aggressive media, wear resistance, low magnetic permeability, non-sparking characteristics, aluminum bronzes can be readily cast, fabricated, and machined [6].
This present study investigated the production of aluminium bronze alloy via sand casting method as compared with the contemporary method of centrifugal casting used in developed countries. The study is aimed at developing a cost effective aluminium bronze alloy with comparable corrosion resistance to those produced abroad and to access the viability of using the materials in a typical Nigerian industrial environment. Findings have shown that aluminum bronze is a fast replacing contemporary steel material for some specific application especially in components for marine/sub-sea applications [7]. This will seek replacement for stainless steel components that fail readily in service as a result of colonization by marine organisms and severe corrosion attack in sea water environment.
The aim of this research work was to study the corrosion behaviour of aluminium bronze alloy suitable for replacing currently used steels in marine and other environments.
Material and Method
Materials for this research work includes aluminium (6063) alloy, copper scraps, sodium chloride (NaCl), sodium hydroxide (NaOH), hydrochloric acid (HCl), sulphuric acid (H2SO4), copper coils and aluminium scraps.
Production of Aluminium Bronze
Charge calculation was carried out to determine the amount of aluminium (6063) ingot and copper scraps to be utilized for the production of the alloy. Predetermined weight of copper scraps was first charged into the diesel-fired pit furnace and heated until melting was achieved at a temperature of 1100°C. Thereafter, predetermined weight of aluminium 6063 ingot was introduced into the melt. The aluminium alloy dissolved immediately in the liquid copper since it has a lower melting point (660°C). The melt was then heated for 2 minutes to achieve proper mixing and fluidity before pouring. Molten aluminium bronze was then poured in already prepared sand mould and was allowed to solidify. As cast rods of 15mm diameter and 100 mm length were produced. The qualities of samples were determined via visual examination and spectrometric analysis using (EDX serial iii X-Ray Fluorescence type).
Immersion Testing
Test samples were cut to 15mm diameter and 10mm length sizes, grinded, polished degreased in acetone and rinsed in water before immersion. Thereafter, the test samples were immersed in HCl, NaCl, NaOH, and H2SO4 media with concentration of 0.1 M, 0.2 M, O.3 M, 0.4 M and 0.5 M respectively. The media were prepared following standard procedures. Weight loss measurement was taken on the samples for a period of 45 days.
Determination of the weight differences
The difference between the initial weight of the samples before immersion and the final weight after immersion was calculated and the result obtained was recorded. It can be expressed mathematically as:
|
W = Wf - Wi |
(1) |
where; W = Total Weight; Wf = Final weight of the specimen; Wi = Initial weight of the specimen
Determination of Corrosion Rate from Weight Measurement
The corrosion rate was obtained using the relation as stated by Fontana, 1986[8].
|
|
(2) |
![]() where: R
= Corrosion rate (mg/mm2/year); W = weight loss/ gain (i.e. weight
difference); A = Area of the specimen; T/365 = Exposure time in days
extrapolated to year (mg/mm2/year)
where: R
= Corrosion rate (mg/mm2/year); W = weight loss/ gain (i.e. weight
difference); A = Area of the specimen; T/365 = Exposure time in days
extrapolated to year (mg/mm2/year)
Determination of Electrode Potential
The electrode potential was determined by the use of DT8300D digital meter. The potential differences obtained were converted to the equivalent value in the standard calomel electrode using equation 3
|
Ezn 1.03V = V (SCE) |
(3) |
where: V= Potential difference; Ezn= Electrode potential difference for Zinc electrode
Reference Electrode
High purity zinc was used as reference electrode for the measurement of potential of the protected steel in sea water.
Results and Discussion
Production Efficiency of Sand Cast Alpha Phase Aluminium Bronze
The results of the chemical composition of Alpha phase aluminium bronze alloy obtained are presented in Table 1 and Figure 1 after the casting of the alloy.
Table 1. Shows the chemical composition of cast aluminium bronze alloy obtained using spectrometer (EDX serial iii X-Ray Fluorescence type) by sand casting.
|
Elements |
Weight [%] |
|
Al |
7.8816 |
|
Cu |
92.0176 |
|
Fe |
0.058 |
|
Zn |
0.0016 |
|
Mn |
0.0024 |
|
Pb |
0.0808 |
From the result, the major elements which constitute the aluminium bronze alloys are the aluminium and the copper elements as expected. The percentage of the aluminium in the alloy was only 7.8816% due to some loss of the aluminum during the casting of the alloy. However, it is still within the range of the alpha phase aluminium bronze alloy which is the major focus of this research work. For the copper content of 92.0176% was obtained after the casting of the alloy. Other alloying elements are iron, zinc, manganese and lead with their percentages.
Table 2 shows the tensile, elongation and the hardness of the aluminium bronze alloys.
Table 2 shows the tensile strength and the Brinell hardness test.
|
Composition |
Tensile strength (N/mm) |
Elongation (%) |
Hardness (BHN) |
|
CuAl8 |
549.3 |
34.5 |
123.1 |
The tensile property was determined using the the Monsanto tensometer while the hardness tests were carried out on the Digital Rockwell Tester using a load of 60 Kgf. The tensile test result obtained was 549N/mm while a hardness test was 123.1BHN and 34.5% was obtained for the elongation of the aluminium bronze alloy.
Figure 1 shows the casting process for the aluminium bronze alloy using the pit furnace method of casting. From the Figure 1 show the various stages where stage i shows the charging of the materials into the pit furnace and follow by stage ii showing the firing of the materials and melting of the charges. Stage iii shows the pouring of the melt into the mould. Stage iv shows the casting process and the cast aluminium bronze produced via sand casting method in accordance with UNS specification CuAl8.
|
|
|
|
Stage i: Charging of the materials into a pit Furnace |
stage ii: Melting of the charge
|
|
|
|
|
Stage iii: Pouring of the melt into the mould cavity |
Stage iv: As cast aluminium bronze
|
Figure 1. Shows the pictorial view of an improvised method of producing aluminium bronze using diesel fired pit furnace
Production efficiency of the process was evaluated by carrying out visual inspections, compositional analysis and mechanical test. The results show that, the hardness of alpha phase aluminium bronze alloy produced have tensile strength and hardness similar to those produce via centrifugal casting reported in literature [9]. Visual examination revealed that alloy castings are free from shrinkage cavities, inclusions, porosities and other casting defects. The visual examination confirms the golden colour and the aesthetic nature of cast aluminium bronze alloy. Compositional analysis revealed that the amount of aluminium present in the castings conforms to the standard range for alpha phase aluminium bonze.
Corrosion Behaviour of Sand Cast Alpha Phase Aluminium Bronze Alloy
|
|
|
|
|
|
(a )Sample in H2SO4 solution |
(b)Sample in NaCl solution |
(c)Sample in NaOH solution |
(d)Sample in HCl solution |
Figure 2 a-2d.Shows the specimen examination after the corrosion test due to corrosion effect on samples of aluminium bronze after 45days of immersion
Figure 3 shows the comparative graph of corrosion rate against the exposure time of Aluminium bronze immersed in 0.1M of various corrosive media.
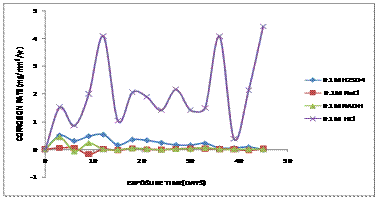
Figure 3.Comparative graph of Corrosion rate (mg/mm2/yr) against Exposure Time (Days) of aluminium bronze immersed in 0.1M of various corrosive media
It was observed that the corrosion rates of samples immersed in acidic solution (HCl and H2SO4 environment) was higher than that of the samples immersed in marine and alkaline solution. The corrosion rates of the sample immersed in 0.1 M of HCl increased to 1.66mg/mm2/yr after 5 days of exposure showing the highest corrosion rate. This was followed by the sample immersed in 0.1M H2SO4 having the corrosion rate of 0.5mg/mm2/yr after 5 days of immersion. High corrosion rates observed in HCl and H2SO4 media is ascribed to high aggressive attack of sulphide and chloride ions on breaking down of the passive film formed on the surface of the aluminium bronze alloy [10]. Sullivan & Wong, [11] reported that the formation of film oxides formed on aluminium bronze consists of a copper-oxide-rich (Cu2O) outer layer and of an alumina-rich (Al2O3) inner layer. Alumina (Al2O3) is easily removed from aluminium bronze at the initial stages and adheres very strongly to a hard copper alloy mating material (known as the counter-face), forming a stable aluminium-rich transfer layer on the alloy and leaving a stable wear resistant copperoxide- rich (Cu2O) film on the aluminium bronze.
A reddish brown colouration was observed to form on the alloy during the 5 days of immersion in the NaOH solution and after 20 days, the NaOH solution changes to blue solution due to reaction of sulphide ions on the copper alloy as shown in figure 2c. The comparative weight loss patterns in the 0.1 M hydrochloric acid solution was observed to be different from those obtained in 0.1 M solutions of NaCl, NaOH and H2SO4 respectively. 0.1M HCl generally showed an increase in corrosion rate with time of immersion in the 0.1M HCl solution forming a green patina colouration deposit due to chlorides attack as shown in figure 2d. However, a constant corrosion rate was observed for the 0.1M NaCl, H2SO4 and NaOH of the alloy which later decreases with immersion time for the 45 days period.
Figure 4 show that aluminium bronze experienced more corrosion attack in hydrochloric acid than sodium chloride, sodium hydroxide and sulphuric acid solutions.
The presence of aluminium oxides was responsible for the decrease in the corrosion rates of both alloys due to the percentage constituents of the aluminium in the alloy forming the protective layer in Figure 2a-2d and Table 1. It was observed that, corrosion rate for sample immersed in 0.2M of HCl solution increases due to attack by the chloride ions after 5days of exposure as shown in the Figure 2b. After 10 days of exposure, there was a significant reduction in the corrosion rate which tends towards passive region. This was because a protective film was formed on the material. After 18 days, there was an increase in corrosion rate as a result of breaking of the protective film formed and exposed the copper alloy to corrosion due to the breaking and removal of the passive film by the chloride attack during pickling/cleaning of the sample for weight loss measurement. The aluminium bronze surfaces must be clean, because any dirt, oil, or grease on the surface will interfere with the chemical action of the solution. Other samples immersed in 0.2M respectively show a low corrosion rate because they tend towards the passive region and thereby a protective film was formed which prevent corrosion to occur for 45 days period.
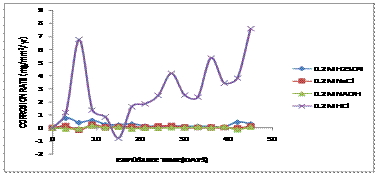
Figure 4.Comparative graph of Corrosion Rate (mg/mm2/yr) against Exposure Time (Days) of aluminium bronze immersed in 0.2M of various corrosive media
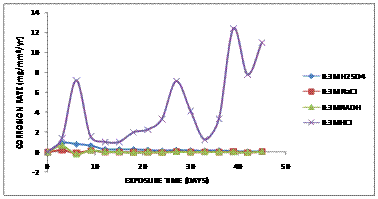
Figure 5.Comparative graph of Corrosion Rate (mg/mm2/yr) against Exposure Time (Days) of aluminium bronze immersed in 0.3M of various corrosive media
Figure 5 shows the comparative graph of corrosion rate against the exposure time of aluminium bronze immersed in 0.3M of various corrosive (HCl, H2SO4, NaOH and NaCl) media. There was a rapid increase in corrosion rate from zero level to 7.15mg/mm2/yr for sample immersed in HCl within the first 2 days of exposure. The corrosion rate later fluctuated by dropping on day 10 and increasing afterwards due to the forming and breaking of the Al2O3 film formed on the surface of the substrates. The films could not protect the aluminium bronze alloy from further chloride attack. However, the corrosion rate for the samples immersed in H2SO4, NaOH and NaCl decreases with the increase in immersion time. This clearly indicates that the protective film formed was able to protect the alloy from corrosion in these environments.
A typical black sulphide film was observed during the cleaning of the samples for weight loss measurement after 5 days of exposure as shown in Figure 2c. Sulfides form a black corrosion product that is less adherent and protective than the normal oxide film. Under susceptible conditions, unwanted pitting or accelerated general corrosion may occur while oxide film on the copper will cause poor adherence of the patina [12]. The film is adherent, protective and generally brown and sometimes greenish brown colour. It was also observed that the black sulphide film gradually turned reddish black colouration on the surface of the samples indicating that a protective scale was form due to cupro oxide form by the copper on reaction with the sulphides and hence increased the corrosion rate.The corrosion rate was observed to reduce and enter passitivity. The film formed was observed to quickly heal up and a partial protective film which further reduced the corrosion rate after 20 days of exposure and linearly continue till 45days of exposure in 0.3M NaCl, H2SO4 and NaOH.
Figure 6 shows the comparative graph of corrosion rate against the exposure time of Aluminium bronze immersed in 0.4M of various corrosive media.
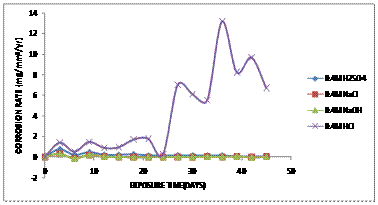
Figure 6. Comparative graph of Corrosion Rate (mg/mm2/yr) against Exposure Time (Days) of aluminium bronze immersed in 0.4M of various corrosive media
From the graph obtained, it was observed that the corrosion rate increases significantly with sample immersed in 0.4M concentration of HCl solution between 0 and 20 days as compared with other samples in other corrosive media with no corrosion owing to the fact that a protective film was formed on the surface of the alloy and thereby protects the alloy from corrosion attack. After 25 days, there was an increase in corrosion rate to about 7.75mg/mm2/yr for sample immersed in HCl solution and later dropped to 6.98mg/mm2/yr. Other samples were found to be in passive region with no corrosion occouring on the samples. During this period, there was a rapid change in the colouration of the solution under normal atmospheric condition to blue and later changes to green in the 25th day of exposure which produced a green patination on the surface of the samples This can be attributed to the chloride attack on the copper alloy forming copper chloride with the reaction with HCl solution [13].
Typical breakdown of the passive film and repassivation of the alloy behaviour was observed in the corrosion rates of both alloys hydrochloric acid solution. Chloride solutions are corrosive because the solution even in small amount usually breaks down the protective film on aluminium bronze [14]. The presence of chlorides in the environment can break down the passive film locally and prevent the reformation of a new film [15].
Figure 7 shows the comparative graph of corrosion rate against the exposure time of aluminium bronze immersed in 0.5M of HCl, H2SO4, NaCl and HCl of various corrosive media.
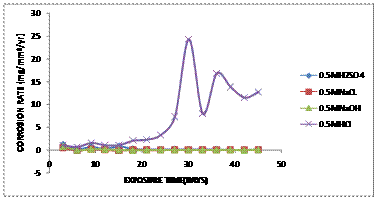
Figure 7. Comparative graph of Corrosion Rate (mg/mm2/yr) against Exposure Time (Days) of aluminium bronze immersed in 0.5M of various corrosive media
From the graph, it was observed that there was a low corrosion rate occouring at the initial immersion into various corrosion media. This observation implies that the specimens are in the passive region and no corrosion was taken place due to the protective film form on the surface of the alloy preventing corrosion to take place. But after 15 days, there was a drastic increase in corrosion rate for sample immersed in 0.5M HCl due to the breakdown of the passive film by chloride attack. And this shows that, the corrosion rate increases with exposure time and concentrations. There was no significant increase in corrosion rate for other samples in H2SO4, NaOH and NaCl solutions.
Conclusions
The research work has shown the corrosion behaviour of aluminium bronze alloy using different selected media (H2SO4, NaCl, HCl, and NaOH).
· The production of alpha phase alumnium bronze is possible and efficient via sand casting.
· Aluminium bronze was more susceptible to corrosion attack in acidic environments as compared to marine and alkaline environments.
· Comparative studies of the aluminium bronze alloy in the selected media shows that the corrosion rates decrease slightly with time and remained constant for all concentrations in marine and alkaline media while it increases in acidic media (HCl, H2SO4).
· The decrease in corrosion rate was due to the formation of a protective layer on the surface of the alloy while the increase observed in acidic media was due to higher chloride and sulphide attack on the alloy which results in breaking of protective film. This protective film can be broken and this will result in an increase in the corrosion rate until the layer is reformed.
· The aluminium bronze alloy experience corrosion rate with an increase in concentrations in acidic media but was lower in marine and alkaline media.
References
1. Copper Development Association, Aluminium Bronze Alloys Corrosion Resistance Guide, 1981, 80, p. 2-15.
2. Copper Development Association, Aluminium Bronze Alloys for Industry, 1986, 83, p. 1-13.
3. Copper Development Association, Aluminium Bronzes Essential for industries, Hemel Hempstead, 1989, 86, p.1-24.
4. Vin C., Aluminum Bronzes Part I & II journal of Metallurgy of Copper and Copper Alloy,, Copper Development Association, 2000, p. 1-10
5. Gozlan E., Bamberger M., Phase Transformations in Permanent-Mould-Cast-Aluminium Bronze, Journal of materials science, 1988, 23(10), p. 3558-3562.
6. Copper Development Association, Welding of Aluminium Bronzes, Boundary way, Hemel Hempstead, 1988, p.85.
7. Lawrence C. B. J., Vimod K. S., Aluminium Bronze Alloys to Improve the System Life of Basic Oxygen and Electric Arc Furnace Hoods, Roofs and Side Vents, Final Report, U. S. Department of Energy (DOE) Information Bridge, 2006, p.22
8. Fontana M.G., Corrosion Engineering, Mc Graw-Hill Series in materials, New York, 1986, 2nd edition, p.2-10.
9. ***, [online]
Available at: http://properties.copper.org/ (accessed 10/08/2013)
10. Bohni H., Localized corrosion of passive metals, 2000, Corrosion Handbook, Review, R.W., John Wiley and Sons, Inc., Canada,2000 p.173-183
11. Sullivan J.L., Wong L.W., Wear of Aluminium Bronze on Steel under Conditions of Boundary Lubrication - Tribol. Int., 1985, p.275-281.
12. Bardal E., Drugli J.M., Corrosion detection and diagnosis, in Materials Science and Engineering, Eolss Publishers, 2004, Vol III, p. 8.
13. Cunat P. J., The Euro Inox handbook of stainless steels. Luxembourg Euro Inox Materials And Application Series, 2000, p.1
14. Arthur H., Guild lines for use of copper alloy in seawater, a voluntary paper submitted for publication, Blacksburg, VA, 1987, p 34.
15. Leffler, B., Stainless steels and their properties, Outokumpu Technical article, 2000, p.45.







