Kinetics of Demineralization of Shrimp Shell Using Lactic Acid
Alewo Opuada AMEH1*, David ABUTU2, Muhammed Tijani ISA1, Umar RABIU1
1 Department of Chemical Engineering, Ahmadu Bello University, Zaria.
2 Department of Chemical Sciences, Federal University Wukari, Taraba State, Nigeria.
E-mails: alewooameh@yahoo.com; abutlericdav@yahoo.com; mtisaz@yahoo.com; umally89@gmail.com.
* Corresponding author: Phone: +2348035600744
Abstract
Shrimp exoskeleton was demineralized using lactic acid and the kinetics of the demineralisation was studied. The residual concentration of calcium after acid treatment was used as a measure of the degree of demineralisation. Kinetic data was obtained using five acid concentrations (0.2, 0.4, 0.6, 0.8 and 1M) and the obtained kinetic data was fitted to the shrinking core model. For all concentrations, the best predictive model was determined to be ash layer diffusion controlled mechanism.
Keywords
Calcium; Demineralization; Lactic Acid; Shrimp; Shrinking Core Model
Introduction
Bio wastes originated from marine food products such as carapace and head of the shrimp, crab and krill are considered to be a rich source of protein, calcium carbonate and chitin [1]. Chitin is a natural polysaccharide composed of N-acetyl-D-glucosamine units and is the second most abundant biopolymer after cellulose [2]. It is biodegradable, biocompatible and nontoxic; therefore, chitin and its deacetylated derivative chitosan, has numerous applications in various fields, e.g. in food, agriculture, cosmetic, biomedicine, textile, water treatment and pharmaceuticals [1, 3-5].
In the processing of shrimp, between 40 and 50% of the total mass is generated as waste. Crustacean shell waste consists mainly of 3040% protein, 3050% calcium carbonate, and 2030% chitin [6-9], with species and seasonal variations [10]. A small part of the waste is dried and utilized [11], while the rest is dumped into the sea, which is one of the main pollutants in coastal areas [12, 13]. The utilization of shellfish waste has been proposed not only to solve environmental problems, but as a waste treatment alternative to the disposal of shellfish wastes [14].
Conventionally, isolation of chitin from marine waste material involves acid treatment to dissolve calcium carbonate (demineralization) followed by alkaline extraction to solubilize proteins (deproteinization) [3]. The conventional demineralization process of crustacean waste is costly and causes environmental problems. Hydrochloric acid is the most commonly used chemical in the demineralization of crustacean waste. The use of this strong acid harms the physiochemical properties of chitin, results in a harmful effluent wastewater and increases the cost of chitin purification process. Percot et al. [15] reported that using HCl for the demineralization of chitin results in detrimental effects on the molecular weight and the degree of acetylating that negatively affects the intrinsic properties of the purified chitin. They elaborated on the importance of the optimization of the extraction process parameters (pH, time, temperature and solids to acid ratio) in order to minimize chitin degradation and bring the impurity levels down to the satisfactory level for specific applications.
Mahmoud et al. [16] reported that the effectiveness of using lactic and/or acetic acids for demineralization of shrimp shells was comparable to that of using hydrochloric acid and other benefits may include (1) organic acids that are less harmful to the environment (2) can preserve the characteristics of the purified chitin and can be produced from low cost biomass such as cheese whey (3) the resultant organic salts from the demineralization process can be used as a food preservative and/or an environmentally friendly de-icing/anti-icing agents. Fermentation of crustacean shell biowaste using microorganisms, which results in the production of lactic acid and protease, has been used in the demineralization of shrimp shell [17-19]. Ameh et al. [21] reported that the demineralization of deproteinized shrimp shell using dilute hydrochloric acid was a chemical reaction controlled process. Owing to the scanty literature on shrimp demineralization kinetics, there is the need to investigate the kinetics of organic acid demineralization of shrimp shell for better control and optimization of the process.
This work investigated the effect of lactic acid on the kinetics of shrimp shell demineralization.
Material and Method
Shrimp was obtained from Kaduna central market and taken to the Department of Biological Science, Ahmadu Bello University for identification. The exoskeleton of the shrimp was manually removed, washed, dried, and ground to pass through a 250µm sieve. Lactic acid (AnalaR, BDH was procured and prepared into five concentrations (0.2, 0.4, 0.6, 0.8 and 1M).
Demineralization
256ml of 0.2M lactic acid solution was introduced into a conical flask (500ml) with stirring on a magnetic stirrer at room temperature (≈ 27oC). 16g of the prepared shrimp shells were quickly introduced and stirred for 5 minutes after which the content of the flask was quickly filtered and washed with deionized water until neutrality as determined using a pH meter (Kent EIL 7055). The demineralized samples were dried and weighed. This was repeated in turns for other reaction times of 10, 15 and 20 minutes.
The entire procedure was then repeated for the other lactic acid concentrations of 0.4, 0.6, 0.8 and 1M.
The concentration of calcium, in the raw as well as demineralized solid materials, was determined using AAS analysis (Atomic Absorption Spectrometer, Varian AA240FS).
Kinetic modeling
The shrinking core model (SCM) was considered as it models fluid-particle reactions [20]. The models for the various SCM control mechanism are:
1. For fluid-film diffusion control (FFDC)
|
t/τ = XB |
(1) |
2. For ash layer diffusion control (ALDC)
|
t/τ = 1 3(1-XB)2/3 + 2(1 XB) |
(2) |
3. For chemical reaction control (CRC)
|
t/τ = 1 (1 XB)1/3 |
(3) |
where τ = time for complete reaction, XB = conversion of Calcium
The right hand side of Equation 1, 2 and 3 were plotted against t and the fit R2 were computed.
Results and Discussion
Figure 1 presents the progression in the concentration of Calcium in the demineralised shrimp shells with time for the various acid concentrations considered.
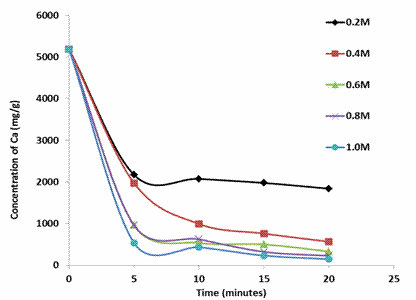
Figure 1. Effect of lactic acid treatment on the Ca content of demineralised shrimp shell
Figure 1 indicates a progressive drop in the concentration of the Ca for all lactic acid concentrations with time. Increasing acid concentration resulted in an increase in the conversion or amount of calcium removed. For instance, after 20 minutes of demineralisation the conversion of Ca for 0.2M and 1.0M lactic acid solutions where 65% and 97% respectively. This is expected owing to the greater reactivity and availability of the acid.
Figures 2 - 5 present the fitting of the kinetic data to the shrinking core model.

Figure 2. SCM for shrimp demineralization using 0.2M acetic acid solution
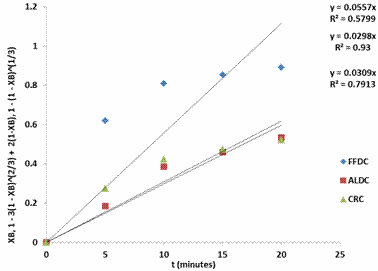
Figure 3. SCM for shrimp demineralization using 0.4M acetic acid solution
Figures 2 - 5 showed that the ash layer diffusion control mechanism (ALDC) gave a better approximation of the demineralization process compared to fluid film diffusion control (FFDC) or chemical reaction control (CRC). The relative magnitudes of the R2 values are indicative of this: for all lactic acid concentrations considered, the R2 values were highest for ALDC models. Furthermore, treatment with 0.4M lactic acid resulted in the highest R2 value of 0.93 for ALDC mechanism. For ALDC reaction it is visualized that a wall of ash (non-reactive materials) prevents the fluid from moving freely to the zone of reaction (that is the unreacted core). In the demineralization of shrimp shells other unreactive materials such as protein and chitin etc are present which can offer this resistance. The result of this investigation is widely different from that reported by Ameh et al. [21] in which it was reported that the CRC mechanism was rate limiting. This again is expected as the investigation of Ameh et al. [21] was based on the demineralization of deprotenised shrimp shell in which case the protein inhibition was no longer existing, having being removed by alkali treatment prior to demineralisation.
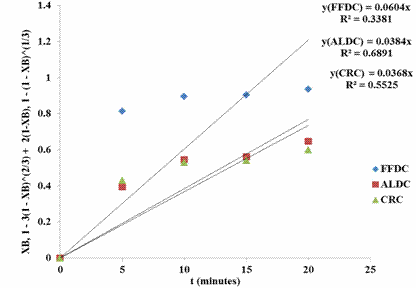
Figure 4. SCM for shrimp demineralization using 0.6M acetic acid solution
Imports of ALDC mechanism include; reaction time is directly proportional to the square of particle size, temperature changes do not have significant effect on the reaction and the ash resistance is unaffected by changes in fluid velocity [20].
The SCM model also supposes a large excess of the reacting fluid, a condition which was not used in this investigation. The generally good fit to the ALDC mechanism suggest that a very good fit may be obtained using excess dilute lactic acid which will provide better kinetic parameters for the purpose of design.

Figure 5. SCM for shrimp demineralization using 0.8M acetic acid solution

Figure 6. SCM for shrimp demineralization using 1.0M acetic acid solution
Conclusion
Shrimp shell was demineralised using 0.2M, 0.4M, 0.6M, 0.8M and 1.0M acetic acid. The concentration of acid used affected the rate and extent of demineralisation. Analysis of the kinetic data using shrinking core model indicated that the reaction was largely ash layer diffusion controlled.
Acknowledgement
The authors wish to acknowledge the Multi-user Research Laboratory, Ahmadu Bello University for allowing the use of the Atomic Absorption Spectrometer.
References
1. Choorit W., Patthanamanee W., Manurakchinakorn S., Use of Response Surface Method for the Determination of Demineralization Efficiency in Fermented Shrimp Shells, Bioresource Technology, 2008, 14(99), p. 6168-6173.
2. Ravi Kumar M.N.V., A Review of Chitin and Chitosan Applications, Reactive and Functional Polymers, 2000, 1(46), p. 1-27.
3. Rinaudo M., Chitin and Chitosan: Properties and Applications, Progress Polymer Science, 2006, 7(31), p. 603-632.
4. Shirai K., Guerrero I., Huerta S., Saucedo G., Castillo A., Obdulia Gonzalez R., Hall G.M., Effect of Initial Glucose Concentration and Inoculation Level of Lactic Acid Bacteria in Shrimp Waste Ensilation, Enzyme Microbial Technology, 2001, 4-5(28), p. 446-452.
5. Kim W.J., Lee W.G., Theodore K., Chang H.N., Optimization of Culture Conditions and Continuous Production of Chitosan by the Fungi, Absidia Coerulea, Biotechnological Bioprocessing Engineering, 2001, 1(6), p. 6-10.
6. Zhang H., Li R., Liu W., Effects of Chitin and Its Derivative Chitosan on Postharvest Decay of Fruits: A Review, International Journal of Molecular Sciences 2011, 12, p. 917-934.
7. Crini G., Guibal E., Morcellet M., Torri G., Badot P.M., Chitin and Chitosan. Preparation, Properties and Main Applications. In: Chitin and Chitosan. Application of Some Biopolymers, University Press of Franche-Comté, Besançon, France, 2009, p. 19-54.
8. Jo G.H., Park R.D., Jung W.J., Enzymatic Production of Chitin from Crustacean Shell Waste. In: Chitin, Chitosan, Oligosaccharides and Their Derivatives, S.K. Kim (Ed.), CRC Press, Taylor and Francis Group, Boca Raton, FL, USA, 2011, p. 37-45.
9. Kurita K., Chitin and Chitosan: Functional Biopolymers from Marine Crustaceans, Marine Biotechnologies, 2006, 8, p. 203-226.
10. Cho Y.I., No H.K., Meyers S.P., Physicochemical Characteristics and Functional Properties of Various Commercial Chitin and Chitosan Products, Journal of Agricultural Food Chemistry, 1998, 46, p. 3839-3843.
11. Xu Y., Gallert C., Winter J., Chitin Purification from Shrimp Wastes by Microbial Deproteination and Decalcification, Applied Microbiological Biotechnologies, 2008, 79, p. 687-697.
12. Zhai X., Hawkins S.J., Interactions of Aquaculture and Waste Disposal in the Coastal Zone, Journal Ocean University, China, 2002, 1, p. 8-12.
13. Gimeno M., Ramírez-Hernández J.Y., Mártinez-Ibarra C., Pacheco N., García-Arrazola R., Bárzana E., Shirai K., One-Solvent Extraction of Astaxanthin from Lactic Acid Frmented Shrimp Wastes, Journal of Agricultural Food Chemistry, 2007, 55, p. 10345-10350.
14. Wang S.L., Chang T.J., Liang T.W., Conversion and Degradation of Shellfish Wastes by Serratia sp. TKU016 Fermentation for the Production of Enzymes and Bioactive Materials, Biodegradation, 2010, 21, p. 321-333.
15. Percot A., Viton C., Domard A., Optimization of chitin extraction from shrimp shells. Biomacromolecules, 2003, 4, p. 12-18.
16. Mahmoud N.S., Ghaly A.E., Arab F., Unconventional Approach for Demineralization of Deproteinized Crustacean Shells for Chitin Production, American Journal of Biochemistry and Biotechnology, 2007, 3(1), p. 1-9.
17. Khorrami M., Najafpour G.D., Younesi H., Amini G.H., Growth Kinetics and Demineralization of Shrimp Shell Using Lactobacillus plantarum PTCC 1058 on Various Carbon Sources, Iranica Journal of Energy and Environment, 2011, 2(4), p. 320-325.
18. Choorit W., Patthanamanee W., Manurakchinakorn S., Use of Response Surface Method for the Determination of Demineralization Efficiency in Fermented Shrimp Shells, Bioresource Technology., 2008, 14(99), p. 6168-6173.
19. Oh K.T., Kim Y.J., Van Nguyen N., Jung W.J., Park R.D., Effect of Crab Shell Size on Bio-Demineralization with Lactic Acid-Producing Bacterium, Lactobacillus Paracasei Subsp. Tolerans KCTC-3074. Biotechnological Bioprocessing Engineering, 2008, 5(13), p. 566-570.
20. Levenspiel O., Chemical Reaction Engineering, 3rd Edition, John Wiley and Sons, New York, 1999, p. 566-576.
21. Ameh A.O., Isa M.T., Adeleye T.J., Adama K.K., Kinetics of Demineralization of Shrimp Exoskeleton in Chitin and Chitosan Synthesis, Journal of Chemical Engineering and Material Science, 2013, 4(3), p. 32-37.