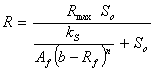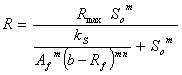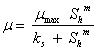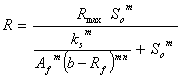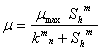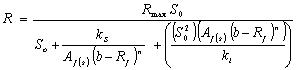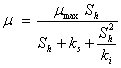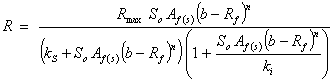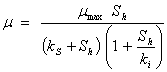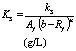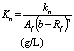Application of Simplified Anaerobic Digestion Models (SADM’s) for Studying the Biodegradability and Kinetics of Cow Manure at Ambient Temperature
Yusuf O.L. MOMOH1* and Benedict ANYATA2
1 Department of Civil and Environmental Engineering, University of Port-Harcourt, Choba, P.M.B 5323 Rivers State, Nigeria.
2 Department of Civil Engineering, University of Benin, P.M.B 1154,
Benin City, Edo State, Nigeria.
E-mails: yusuf.momoh@uniport.edu.ng; bufanyat_a@yahoo.co.uk
* Corresponding author: +2348035386779
Abstract
The application of a set of simplified anaerobic digestion models (SADM’s) to describe the anaerobic biodegradability and kinetics of cow manure at ambient temperature was conducted in this study. It was observed that the Hill’s based biogas yield rate model was the most appropriate in describing biogas yield rate from cow manure. Parameter estimation revealed that the half saturation constant expressed as acidified substrate and volatile solids (VS) equivalent were 0.163g/l and 21.9g VS/l respectively while the maximum biogas yield rate was estimated to be 1.957ml/g VS/day. The coefficient of acidogenic bacteria adaptation (n) and coefficient of acetogenic/methanogenic bacterial cooperativity (m) were estimated to be 1.28 and 0.65 respectively. The poor cooperativity amongst the acetogenic/methanogenic bacterial species can be attributed to poor adaptation, possibly due to interaction between ammonia and volatile fatty acids. In addition, the biodegradability and recalcitrance was estimated to be 0.42 and 0.433 respectively, while hydrolysis/acidogenesis was identified as the rate limiting step.
Keywords
Anaerobic; Biogas Yield; Cow Manure; Ambient Temperature; Biodegradability; Saturation Constants; Kinetic Models; Performance.
Introduction
In an attempt to improve the living standard of developing countries, one hundred and eighty nine countries (189) countries met at the United Nations (UN) headquarters in September 2000 and agreed on an eight goal communiqué called the millennium development goals (MDG’s). These goals comprised (i) eradication of extreme poverty and hunger; (ii) achieve universal primary education; (iii) promote gender equality and empower women; (iv) reduce child mortality (v) improve maternal health; (vi) combat HIV/AIDS, malaria and other diseases; (vii) ensure environmental sustainability and (viii) achieving global partnership for sustainable development [1]. These goals which are expected to be met by 2015 have been faced with series of challenges especially the problems of inadequate energy sources, a fact which has been identified by Mshandete and Parawira [2] who recognized the availability of sustained energy access as a major prerequisite for attainment of the millennium development goals especially in sub-Saharan Africa.
Globally, more than 1.6 million people live without access to electricity while majority of the poor world live in sub-Saharan Africa with about 60-70% of the most populous sub-Saharan country having no access to electricity thus, relying strongly on wood fuel as the predominant energy source [3,4]. Because of the relevance of energy access in the attainment of the millennium development goals, the need to diversify energy sources has received encouragement in many developing countries and the utilization and adoption of domestic biogas technology has been identified as having the potentials of supporting energy services and also contributes to meeting the needs of the millennium development goals [2].
Domestic biogas technology produces useful fuel (biogas) rich in methane that can be utilized for cooking and heating purposes thus, contributing to hunger eradication. Also, the bio-slurry is a potent organic fertilizer which can reduce the use of chemical fertilizer thus, contributing to improve agricultural production and food security. In the area of sanitation, domestic biogas technology can promote environmental sustainability by reducing environmental pathogens and encouraging conservation of resources which generally support the ideas of the millennium development goal.
Traditionally, cow manure is the predominant feedstock used in rural community for domestic biogas production [5]. Though, cow manure is rich in methanogens necessary for methane production, its high lignin content and nitrogen content tend to militate against effective biogas production. Also, it has been reported that, the digestion of cow manure results in a complex biochemical interaction of free ammonia, volatile fatty acids which are both by product of the anaerobic process that produces an “inhibited steady state” phenomenon. In this condition, anaerobic digestion proceeds stably but with lower methane yield [6]. In addition to this phenomenon, ammonia has been established to inhibit acetoclastic methanogenesis. Gallert and Winter [7] reported that free ammonia of 560–568 mg NH3 N/l caused a 50% inhibition of methanogenesis at pH of 7.6 under thermophilic condition. Another study on cattle manure at thermophilic temperature also indicated that free ammonia above 700 mg NH3 N/l resulted in a poor treatment performance at a pH of 7.4–7.9 [8].
Thus, in order to appropriately understand the anaerobic degradation kinetics of cow manure, adequate model testing and validation is required. Angelidaki [9] reported that many of the existing substrate utilization models that are based on Monod growth models are incapable of describing the kinetics of biogas production from cow manure and afterwards developed a complex model that accounted for hydrolysis, acidogenesis, acetogenesis, methanogenesis, free ammonia and volatile fatty that could appropriately describe the kinetics of biogas production from cow manure. Moreover, with the development of anaerobic digestion model No. 1(ADM 1), researchers have been able to evaluate the anaerobic digestion of cow manure, though with some complications and alteration of the default kinetic parameter associated with ADM 1 [10].
The utilization of complex models to assess anaerobic digestion of cow manure can be very complicated and time consuming, hence, the need for simplified, generalized models for evaluating kinetics of biogas production from complex biomass cannot be overemphasized. Although, simplified substrate utilization kinetic models based on Contois growth model have previously been employed for assessing the kinetics and biodegradability of cow manure [11, 12], these models depended strongly on the ability to measure the bacteria biomass volatile suspended solids which can be very difficult procedure because of the difficulty in differentiating between bacteria biomass volatile solids and complex biomass volatile solids [13]. In this research, the application of simplified kinetic models as developed by Momoh et al. [14] was applied in studying the kinetics and biodegradation of cow manure.
The aim of the research was focused on determining the kinetics and biodegradability parameters of cow manure undergoing anaerobic digestion at ambient temperature conditions using a set of simplified anaerobic digestion models (SADM’s). These parameters would provide sufficient information about the anaerobic bacterial behaviour and characteristic of cow manure utilized in anaerobic digestion.
Material and Method
Cow manure utilized in this research was obtained from abattoir situated at Choba community of Rivers State, Nigeria. About 1000g of cow manure was sun dried for 20days to facilitate its measurement. A weighing balance (Mettler, model PN163) manufactured in Switzerland with specification range between 0.1mg and 160g was utilized for mass measurements. Crushing of cow manure using mortar and pestle preceded weighing of the cow manure. The crushed cow manure was loaded into batch digesters (buchner flasks) labeled A1, A2, A3, A4, A5, A6, A7 and A8 comprising total solid concentration of 2, 3, 4, 5, 6, 7, 8 and 9% respectively. The digesters were set-up as described by Momoh and Nwagoazie [15] and set-ups were conducted in duplicates. The volatile solids content of the cow manure were determined before the digestion process commenced according to APHA [16] using a muffle furnace, Carbolite model LMF 4 manufactured in England. Similarly, the carbon to nitrogen ratio of the feedstock was determined in accordance with APHA [16].
The cow manure was subsequently loaded into Buchner flasks and corked to exclude air as described by Momoh and Nwagoazie [15]. The digesters were allowed to run anaerobically for a period 35 days and agitated twice daily at an average ambient temperature of 28±30C. Water displacement method was used to measure biogas produced. The displaced water was saturated brine solution which prevented the dissolution of carbon dioxide in the water while, biogas produced was analyzed for methane content using Gas Chromatography Agilent Technologies Model 1890A.
In this study, the application of a family of simplified anaerobic digestion models (SADM’s) as developed by Momoh et al., [14] in studying anaerobic digestion of cow manure was conducted. The developed biogas yield rate models of Monod, Moser, Hills, Andrews (Haldane) and Non-competitive Haldane’s were applied in studying the biodegradation and kinetics of biogas production from cow manure at ambient temperature. The mathematical representation of these models is as shown in Table 1. The various model parameters were evaluated using the solver function of the Microsoft Excel tool Pack and the most appropriate model was selected based as its high correlation coefficient and low root mean square error (RMSE) established between the experimental data and the predicted specific biogas yield rate.
The highlight of these set of models, reside in their ability to use numeric values to predict the rate limiting step of anaerobic process using the rate limiting coefficient (Af ). Values of this coefficient greater than 0.5 implied that acetogenesis/methanogenesis was the rate limiting step while, values of the coefficient less than 0.5 implied that hydrolysis/acidogenesis was the rate limiting step [14].
Results and Discussion
The volatile solids content and carbon/nitrogen ratio as determined by standard methods [16] was determined to be 66% and 22:1 respectively while, the anaerobic digestion of the of the digesters contents lasted for a period of 35days after an initial lag phase in all digesters. The methane content and biogas yield rate was observed to increase with increase solid loading ranging from 2-9% (Figure 1 and Table 2). Also, the result from parameter estimation for the five set of models tested in this study is presented in Table 3.
Table 2. Digester characteristics and biogas composition
|
Digester |
Percent (%) Total Solids |
Conc. Volatile solids(g/l) |
pH |
Cumulative biogas(mL) |
CH4 (%) |
CO2 (%) |
|
A1 |
2 |
13.48 |
7.30±0.03 |
94.36±122 |
07±3 |
93±3 |
|
A2 |
3 |
20.43 |
7.35±0.02 |
173.4±10 |
14±3 |
86±3 |
|
A3 |
4 |
27.52 |
7.37±0.04 |
264.9±12 |
26±4 |
74±4 |
|
A4 |
5 |
34.76 |
7.38±0.02 |
341.3±9 |
32±3 |
68±3 |
|
A5 |
6 |
42.16 |
7.40±0.02 |
423.5±13 |
44±2 |
56±2 |
|
A6 |
7 |
49.76 |
7.60±0.03 |
516.4±17 |
48±3 |
52±3 |
|
A7 |
8 |
57.36 |
7.62±0.02 |
647.5±12 |
50±4 |
48±4 |
|
A8 |
9 |
65.38 |
7.68±0.02 |
772.9±10 |
51±4 |
49±4 |
Table 1. Simplified biogas yield rate models (SADM’s) and corresponding growth models
|
Biogas yield rate model |
Biogas yield rate model Equations |
Kinetic growth model Equations |
|
Monod’s based |
|
|
|
Moser’s based |
|
|
|
Hill’s based |
|
|
|
Haldane (Andrews) based |
|
|
|
Non-competitive (Haldane) based |
|
|
|
where, Af = rate limiting step coefficient for fast substrate utilization; As = rate limiting step coefficient for very slow substrate utilization; Af(s) = rate limiting step coefficient for fast or very slow substrate utilization; b = fraction of initial volatile solids remaining in effluent; ks = Monod’s half saturation constant for acidified substrate (g/l); Ks = Monod’s half saturation constants in volatile solids equivalents (g/l); kn = Hill’s half saturation constant for acidified substrate (g/l); Kn = Hill’s half saturation constant in volatile solids equivalents (g/l); ki = substrate inhibition constant for acidified substrate (g/l); m = coefficient of acetogenic/methanogenic bacteria adaptation for cooperativity; n = coefficient of acidogenic bacteria adaptation for complex substrate degradation; Rf = recalcitrant fraction; Rmax = maximum specific biogas yield rate (mL/g VS/day); R =specific biogas yield rate (mL/g VS /day); So = initial volatile solids concentration (g/l); Sh = concentration of acidified substrate generated (g/l); µ = bacteria growth rate (/day); µmax = maximum bacteria growth rate (/day) µmax = maximum bacteria growth rate (/day); VS= volatile solids |
||
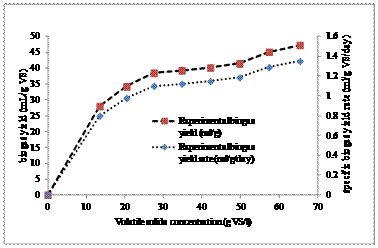
Figure 1. Experimental biogas yield and rate production with volatile solids concentration
Table 3. Parameter estimate for developed biogas yield rate models
|
Biogas yield rate models |
Rmax mL/gVS/day |
ks g/l |
kn (g/l) |
ki (g/l) |
KS |
Kn |
m |
n |
Rf |
b |
Af(s) |
RMSE |
|
Monod based |
1.54 |
0.699 |
- |
- |
43 |
- |
- |
1.48 |
0.383 |
0.461 |
0.177 |
9.59E-03 |
|
Moser’s based |
1.957 |
0.41 |
- |
- |
34 |
- |
0.657 |
1.839 |
0.353 |
0.618 |
0.135 |
8.71E-03 |
|
Hill’sbased |
1.957 |
- |
0.163 |
- |
- |
21.9 |
0.657 |
1.28 |
0.433 |
0.578 |
0.088 |
8.71E-03 |
|
Haldane (Andrew) based |
1.54 |
1.8E-07 |
- |
2.6 |
11.23 |
- |
- |
2.33 |
0.487 |
0.513 |
7.9E-09 |
9.59E-03 |
|
Non-competitive Haldane based |
1.54 |
5.6E-06 |
- |
373.6 |
12.4 |
- |
- |
0.012 |
0.414 |
0.624 |
4.6E-07 |
9.59E-03 |
|
|
||||||||||||
A set of simplified anaerobic digestion models (SADM’s) were used to describe the kinetics and biodegradability of cow manure and the parameters that were estimated or evaluated to include the following;
(a) Monod half saturation constant for the hydrolyzed and acidified substrate (ks) (g/l).
(b) Monod half saturation constant in volatile solids equivalent (Ks) (g/l).
(c) Hill’s half saturation constant for the acidified substrate (kn) (g/l).
(d) Hill’s half saturation constant in volatile solids equivalents (Kn) (g/l).
(e) Maximum specific biogas yield rate (Rmax) (mL/g VS/day).
(f) The coefficient “m”
(g) The coefficient “n”
(h) Fraction of volatile solid remaining in effluent (b)
(i) The recalcitrant fraction (Rf).
(j) Fraction of biodegradable volatile solids (1-Rf)
(k) Fraction of biodegradable volatile solids remaining in effluent (b-Rf)
(l) Biodegradability (1-b)
(m) Rate limiting coefficient for fast or very slow uptake of acidified substrate (Af(s))
The kinetic and biodegradability parameters were evaluated using non-linear regression implemented via the Solver function of the Microsoft Excel tool pack and the results for the estimated parameters are presented in Table 2. The appropriateness of a particular model in describing the anaerobic process was reflected on the model’s ability to produce a high correlation coefficient and low root mean square error (RMSE).
Although the five models tested provided correlation coefficient of 0.996 each, the Moser and Hill’s based biogas yield rate models showed lower root mean square errors (RMSE) of 0.00871 each, when compared to other models. Thus, the Moser and Hill’s based biogas yield rate models were selected as most appropriate to describe biogas production rate from cow manure at ambient temperature condition. Hence, further discussions were limited to the Moser and Hills’ biogas yield rate models.
It is important to note that the growth models of Moser and Hill’s are homologue that incorporates a coefficient “m” which differentiates them from the Monod’s growth model. However, the developed Moser and Hill’s based biogas yield rate models have in addition another coefficient “n”. The term “m” is defined as coefficient of adaptation for cooperativity by the acetogenic/methanogenic bacteria, while, the coefficient “n” which is described as the degree of adaptation for complex biomass degradation by the hydrolytic/acidogenic bacteria [14]. For values of m and n greater than unity, some level of positive adaptation for degradation or cooperativity is implied, while values less than unity indicate low level of adaptation for degradation or cooperativity.
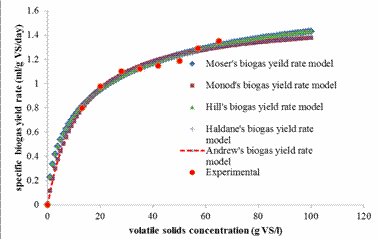
Figure 2. Combined graphs of specific biogas yield rate against volatile solids concentration
Thus, by considering the Moser’s biogas yield rate model, the Monod half saturation constants for the acidified substrate (ks) and the Monod half saturation constant in volatile solid equivalent (Ks) were estimated to be 0.41 and 34g/l respectively. This estimated Monod half saturation constant for the acidified substrate (ks) is consistent with the half saturation constant range of 0.1 - 0.41g/l displayed by acetoclastic methanogens reported by Pavlostathis and Giraldo & Gomez [17]. Furthermore, by considering the Moser’s biogas yield rate model, the biodegradability parameters estimated revealed that the recalcitrant fraction in this biomass mixture was 0.353 of the initial volatile solids fed, and the biodegradable fraction (1-Rf) was 0.647 of the initial volatile solids fed. The biodegradability (1-b) was 0.382 while the biodegradable fraction remaining (b-Rf) was 0.265 of the initial volatile solids fed at ambient temperature conditions.
The coefficients of adaptation for degradation by acidogenic bacteria (n) when considering the Moser based biogas yield rate models were 1.839 and because “n” was greater than unity, some degree of acidogenic bacterial adaptation for degrading the complex biomass was implied. The coefficient of adaptation for cooperativity by the acetogenic/methanogenic bacterial (m) was estimated to be 0.657. However, because the value for “m” was less than unity, the degree of adaptation for cooperativity by the acetogenic/methanogenic bacteria was very poor. The reason for the poor cooperativity observed by the acetogenic/methanogenic bacteria may not be far-fetched from the established problem of ammonia inhibition usually associated with digestion of cow manure. Ammonia which is produced during degradation of cow manure has been implicated in inhibiting methanogenesis [8]. In addition, the rate limiting step for the anaerobic process by considering the Moser’s based biogas yield rate model was estimated to be 0.135 which was lower than 0.5 hence, hydrolysis was suggested to be the rate limiting step. This is consistent with the report of Eastman and Ferguson [18] that considered hydrolysis as rate limiting step for complex biomass.
However, by considering the Hill’s based biogas yield rate model, the Hill’s half saturation constant for the hydrolyzed and acidified substrate (kn) was estimated to be 0.163. This estimated Hill’s half saturation constant for the hydrolyzed and acidified substrate (kn) compares reasonably with values of 0.143-0.207g/l reported by Barthakur et al., [11] for half saturation constant for acetate by the methanogenic bacteria population. Also, this value lies within the range of 0.1- 0.41g/l reported by Pavlostathis and Giraldo, Gomez [17] as half saturation constant displayed by acetoclastic methanogens. In addition, the Hill’s half saturation constant in volatile solid equivalents was evaluated to be 21.9g VS/l which compares reasonably to the value of 24gCOD/l and 26g VS/l reported by Ghaly et al., [19] and Bartharkur et al., [11] for cow manure respectively.
Moreover, by considering the Hill’s based biogas yield rate model, the recalcitrant fraction (Rf) was estimated to be 0.433 which is close to 0.400 reported by Barthakur et al. [11] and Hashimoto [20] for cow manure. In addition, the biodegradable fraction (1-Rf) was calculated to be 0.567 of the initial volatile solids fed while the biodegradability (1-b) was calculated to be 0.422 which is close to 0.46 for the biodegradability of cow manure reported by Hussain [21] while the biodegradable fraction remaining in the effluent (b-Rf) was calculated to be 0.145 of the initial volatile solids concentration.
In addition, the coefficient of adaptation for degradation by acidogenic bacteria (n) when considering the Hill’s based biogas yield rate models was 1.28, while the coefficient of adaptation for cooperativity by the acetogenic/methanogenic bacterial (m) was estimated to be 0.657. Again, because “n” was greater than unity, some degree of acidogenic bacterial adaptation for degrading the complex biomass was implied. However, the value for “m” was less than unity implying that the degree of adaptation for cooperativity by the acetogenic/methanogenic bacteria was very poor. Similar reasons as discussed earlier may be attributed to this low value of “m”.
Moreover, the rate limiting step for the anaerobic process considering the Hills based biogas yield rate model was estimated to be 0.088 which was lower than 0.5. Thus, hydrolysis was suggested to be the rate limiting step which is consistent with the report of Eastman and Ferguson [18] that considered hydrolysis as rate limiting step for complex biomass.
Application of Maximum Specific Biogas Yield Rate (Rmax) for further Model Selection
The models of Moser and Hills biogas yield rate model has been established to be most appropriate for modelling biogas production rate from cow manure at ambient temperature conditions both producing an estimate of 1.957ml/g VS/day as maximum biogas yield rate (Rmax) which is close to value of 1.75ml/g VS/day reported by Budiyono et al., [22] for anaerobic digestion of cow manure at room temperature. However by considering definition of half saturation constant, it was observed that further model selection could be made by comparing the half saturation constant obtained from the non-linear regression and that obtained from graphical definition of half saturation constant.
By definition, the half saturation constant is described as substrate concentration corresponding to 0.5Rmax. From Figure 3, it was observed that the substrate concentration corresponding to 0.5Rmax was 22gVS/l which was similar to the half saturation constant of 21.9gVS/l in volatile solids equivalent obtained by considering the Hill’s based biogas yield rate model. Thus, the Hill’s based biogas yield rate model was selected over the Moser’s biogas yield rate model which estimated the half saturation constant in volatile solids equivalent to be 34gVS/l.
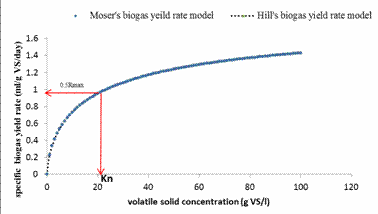
Figure 3. The Moser and Hill’s biogas yield rate plots against volatile solids concentration
Performance Evaluation of Anaerobic Process
The process of digestion of cow manure is usually complicated because of the problem free ammonia inhibition of acetogenesis/methanogenesis. Hence, anaerobic digestion of cow manure is most likely to proceed sub-optimally operating below 100%. An attempt to evaluate the performance for the digestion of cow manure at ambient condition was conducted by plotting of a graph of (R/Rm) versus initial volatile solids substrate concentrations at varying rate limiting step (Af) using the Hills based biogas yield rate model as shown in Figure 4. It is clear from Figure 4 that the efficiency of the anaerobic process at total solids concentration of 9% corresponded to 66% for rate limiting step coefficient of 0.088 estimated in this study. In essence, the expected biogas yield rate at this solid loading would be 0.66*Rmax or 1.30 ml/g VS/day which correspond to that obtained experimentally.
In addition, from Figure 4, it was observed that as the value of the rate limiting coefficient (Af) increased, the performance of the anaerobic process improved. In essence, conditions which increase the rate limiting coefficient (Af) that is, improve the hydrolysis rate (e.g. pulverization) can lead to improvement in performance of anaerobic process especially for volatile solid concentration ranging from 20-100gVS/l. This finding is consistent with that reported by Palmowski et al., [23] who observed an increase in biogas yield during mechanical treatment of complex biomass.
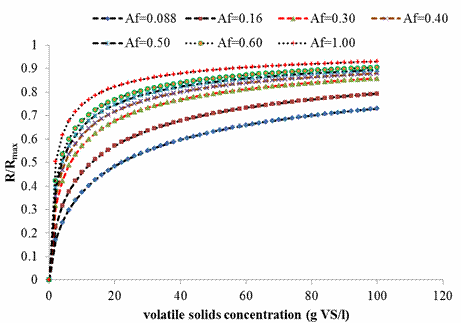
Figure 4. Plot of efficiency of anaerobic process against volatile solids
Conclusions
In this study, the anaerobic breakdown of cow manure and biogas yield production rates were effectively described using the simplified anaerobic digestion models, however, only the Hills based biogas yield rate model was ultimately selected as most appropriate for describing the anaerobic biodegradability and kinetics of cow manure at ambient temperature. The recalcitrant fraction in cow manure was estimated to be 0.433, the biodegradable fraction 0.578; while the biodegradability of cow manure was estimated to be 0.422. The half saturation constant in volatile solids equivalent was determined to be 21.9g VS/l. In addition, the rate limiting step coefficient was determined to be 0.088 which implied hydrolysis/acidogenesis as the rate limiting step.
Acknowledgements
This research was supported by the Petroleum Technology Development Fund Local Scholarship Scheme (Grant Number PTDF/TR/lS/MOLY/215/54) Nigeria.
References
1. Barnes A., Brown G.A., The Idea of Partnership within the Millennium Development Goals: Context, Instrumentality and the Normative Demands of Partnership, Third World Quarterly, 2011, 32(1), p.165-180.
2. Mshandele A.M., Parawira W., Biogas Technology Research in Selected Sub-Saharan. A Review, African Journal of Biotechnology, 2009, 8(2), p.116-125.
3. Uyigue E., Agho M., Edevbaro A., Promoting Renewable Energy and Energy Efficiency in Nigeria, Conference held at the University of Calabar Hotel and Conference Centre 21st November, 2007.
4. Emmanuel E.E., The Prospects of Biofuels in Complimenting the Nigeria’s Energy Needs, International Journal of Environment and Bioenergy, 2012, 4(2), p.74-85.
5. Ramachandra T.V., Geographical Information System Approach for Regional Biogas Potential Assessment, Research Journal of Environmental Science, 2008, 2(3), p. 170-184.
6. Angelidaki I., Ahring B.K., Thermophilic Digestion of Livestock Waste: The Effect of Ammonia, Applied Microbiology Biotechnology, 1993, 38, p. 560-564.
7. Gallert C., Winter J., Mesophilic and Thermophilic Anaerobic Digestion of Source-Sorted Organic Waste: Effect of Ammonia on Glucose Degradation and Methane Production. Applied Microbiology Biotechnology, 1997, 48, p. 405-410.
8. Angelidaki I., Ahring BK., Anaerobic Digestion of Manure at Different Ammonia Loads: Effect of Temperature, Water Resources, 1994, 28, p. 727-731.
9. Angelidaki I., Anaerobic Thermophilic Biogass Process: the Effect of Lipids and Ammonia, Ph.D. Thesis, Copenhagen, The Technical University of Denmark, 1992.
10. Lubken M., Wichern M., Schlattmann M., Gronauer A., Horn H., Modelling the Energy Balance of an Anaerobic Digester Fed with Cattle Manure and Renewable Energy Crops, Water Research, 2007, 41(18), p. 4085-4096.
11. Bartharkur A., Bora M. Singh, H.D., Kinetic Model for Substrate Utilization and Methane Production in the Anaerobic Digestion of Organic Feeds, Biotechnology Programme, 1991, 7(4), p. 369-376.
12. Bala B.K., Satter M.A., Kinetic and Economic Consideration of Biogas Production Systems, Biological Waste, 1990, 34, p. 21-38.
13. Shanmugam P., Horan N.J., Simple and Rapid Methods to Evaluate Methane Potential and Biomass Yield for a Range of Mixed Solid Wastes, Journal of Bioresources Technology, 2008, 100, p. 471-474.
14. Momoh O.L.Y., Anyata B.U., Saroj D.P., Development of Simplified Anaerobic Digestion Models (SADM’s) for Studying Anaerobic Biodegradability and Kinetics of Complex Biomass, Biochemical Engineering Journal, 2013, 79, p. 84-93.
15. Momoh O.L.Y., Nwaogazie I.L., The Effect of Waste Paper on the Kinetics of Biogas Yield from the Co-Digestion of Cow Manure and Water Hyacinth, Biomass Bioenergy, 2011, 35, p. 1345-1351.
16. APHA, AWWA, WPCE, Standard Methods for the Examination of Water and Wastewater, 16th Ed. APHA, Washington D.C, 1985.
17. Pavlostathis S.G., Giraldo-Gomez E., Kinetics of Anaerobic Treatment. A Critical Review, Critical Reviews and Environmental Control, 1991, 21(5-6), p. 411-490.
18. Eastman J.A, Ferguson J.F., Solubilization of Particulate Organic Carbon During the Acid Phase of Anaerobic Digestion, Journal of Water Pollution Control Federation, 1981, 53, 352-366.
19. Ghaly A.E., Sadaka S., Hazza’a S., Kinetic of an Intermittent Flow, Continuous-Mix Anaerobic Reactor, Energy Sources, 2000, 22, 525-542.
20. Hashimoto A.G., Methane from Cattle Waste: Effects of Temperature, Hydraulic Retention Time, and Influent Substrate Concentration on Kinetic Parameter (k), Biotechnology Bioengineering, 1982, 24, 9, p. 2039-52.
21. Husain A., Mathematical Models of the Kinetics of Anaerobic Digestion-a Selected Review. Biomass Bioenergy, 1998, 14, p. 561-571.
22. Budiyono I.N., Widiasa S., Johari, Sunarso, The Kinetics of Biogas Production Rate from Cattle Manure in Batch Mode, International Journal of Chemical and Biological Engineering, 2010, 3(1), p. 39-44.
23. Palmowski L., Muller J., Anaerobic Degradation of Organic Materials-Significance of the Substrate Surface Area, Water Science and Technology, 2003, 47(12), p. 231-238.