The Inhibition Effect of Eugenol to the Biocorrosion of Titanium in Saliva Medium
Latifa KINANI1, Abdelilah CHTAINI1, H. LATRACHE2
1 Molecular Electrochemistry and Inorganic Materials Team, Faculty of Sciences and Technology, Beni Mellal, Maroc.
2 Laboratoire de Bioprocédés et Bio-interfaces, Département de Science de la Vie, Faculté de Sciences et Techniques, Université Sultan Moulay Slimane, B.P. 523 Béni Mellal, Maroc.
E-mails: latifa_kinani@yahoo.fr, chtainia@yahoo.fr; latracheh@yahoo.fr
*Phone: 0618316382
Abstract
The inhibition efficiency of eugenol in controlling corrosion of titanium grade 2 in saliva medium containing bacteria at different pH has been evaluated by electrochemical polarization methods, and electrochemical impedance spectroscopy. The electrochemical data show that the corrosion resistance is greatly enhanced after surface modification. The best protection is obtained with eugenol at pH 7. The Scanning electron microscopy analysis showed that theses inhibitors act by establishment of a thin film at the metal surface. The film, act as a barrier to the transport of the metal ions from the metal to the solution at high concentration of inhibitor acts by establishment of a thin film at the metal surface.
Keywords
Corrosion; Inhibition; Saliva Medium; Eugenol; Bacteria; pH; Dental Alloys.
Introduction
The deterioration of metal due to microbial activity is termed biocorrosion or microbially influenced corrosion. Wing to its economic and environmental importance; microbially influenced corrosion has been the subject of extensive studies for the past five decades and several models have been proposed to explain mechanisms governing biocorrosion [1].
Bacteria are considered the primary colonizers of inanimate surface in saliva medium; the majority of microbially influenced corrosion investigations have addressed the impact of pure or mixed culture bacterial biofilms on corrosion behavior of iron; copper; aluminium and their alloys. The main types of bacteria associated with metals in terrestrial and aquatic habitats are sulfate-reducing bacteria and bacteria secreting organic acids and slime [2]. These organisms typically coexist in naturally occurring biofilms forming complex consortia on corroding metal surfaces [3-5].
An important method of protecting materials against deterioration from corrosion is by using inhibitors [6-8]. The corrosion inhibition of iron and its alloys by different inhibitors in acidic medium has been studied by several authors [9].
The objective of this study was to demonstrate the effect of eugenol on the titanium corrosion in artificial saliva at different pH. The result shows that eugenol is a better inhibitor of bacterial corrosion of the titanium G2 in the both medium at high concentration.
Material and Method
The used specimens were plants (1cm x 1cm x 1mm) placed in different medium, adaptable to the working electrode. The samples were mechanically cleaned with abrasive strips. The electrolyte reference used was Fusayama Meyer artificial saliva. The composition of this solution, which closely resembles natural saliva, is KCl (0.4 g/l), NaCl (0.4 g/l), CaCl2. 2H2O (0.906 g/l), NaH2PO4 2H2O (0.690 g/l), Na2S 9H2O (0.005 g/l), and Urea (1 g/l). The pH was measured with an XC type glass electrode. The first test medium is the saliva at pH=7.03.The second medium used had the same contents as the first, but the pH was lowered by adding lactic acid. This acid was chosen in order to obtain conditions that were as close as possible to the clinical reality. The third medium was identical to the reference medium but was enriched with bacteria and the last medium was containing bacteria at pH 2 at different concentration of eugenol.
For electrochemical step, a glass electrochemical cell was used, with the thermostat set at 37 ± 0.1°C. The three electrodes system was used, with a saturated calomel electrode, platinum plat (1cm x 1cm) counter electrode and working electrode (specimens test) connected to a Volta lab 10 type computer controlled potentiostat. The electrochemical analysis involved, measuring of linear polarization resistance (Rp), potentiodynamic polarization and AC impedance in different media. The surfaces of the specimens were observed on scanning electron microscopy.
The bacterial strain used in this study was Staphylococcus aureus ATCC 25923. The strain was cultured overnight at 37°C in Luria–Bertani agar containing the following (per liter of distilled water): 10 g of tryptone, 5 g of yeast extract 10 g of NaCl and 15 g of agar. Then, the bacterial cells were harvested by centrifugation for 15 min at 8400 x g and were washed twice with, and resuspended in saliva medium solution with. The concentration of bacterial suspensions was adjusted by measuring the initial DO at 405 nm (DOi = 0.7 to 0.8) which corresponds to 108 CFU/ ml.
Results and Discussion
Figure 1 and Figure 2 shows the cathodic and anodic polarisation curves of titanium grade 2 in saliva medium containing bacteria at different pH with and without addition of eugenol.
Table 1 and table 2 gives the values of the associated electrochemical parameters. The corrosion inhibition efficiency (E%) was calculated by:
|
E% = [(Icorr – I’corr) / Icorr] × 100 |
(1) |
where Icorr and I’corr are the uninhibited and inhibited corrosion current densities, respectively, determined by extrapolation of the cathodic and anodic Tafel lines to corrosion potential (Ecorr).
As can be seen from Figure 1, cathodic and anodic current-potential curves give rise to parallel Tafel lines indicating that the hydrogen evolution reaction is activation controlled and the addition of the inhibitors studied does not modify the mechanism of this process. The value of the corrosion potentials (Ecorr) is modified by the addition of eugenol compounds. The addition of the compounds studied decreases the current densities in a large domain of anodic and cathodic potentials. This result indicates that eugenol compounds acts as anodic and cathodic inhibitors. The decrease in Icorr was pronounced with eugenol (Table 1).
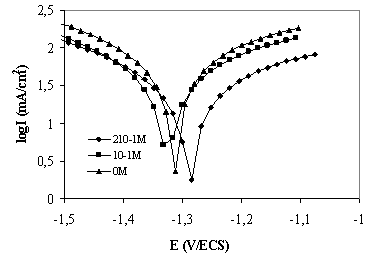
Figure 1. Polarisation curves of titanium G 2 in saliva medium at pH=7 containing bacteria at with eugenol inhibitor at different concentrations, Intensity curve potential
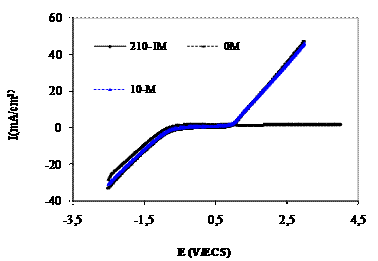
Figure 2.Polarisation curves of titanium grade 2 in saliva medium at pH=2 containing bacteria at with eugenol inhibitor at different concentrations
The electrochemical parameters determined from the polarization curves (Figure 1 and Figure 2) are given in Table 1 and Table 2. The corrosion current density of titanium grade 2 increased with increasing of pH, from 53.30 µA/cm2 in reference medium containing bacteria, to 111.99AµA/cm2 in pH= 2 medium containing bacteria. The corrosion current density decreased with the increasing concentration of eugenol in the both medium, from 8.10µ A/cm2 in reference medium containing bacteria and eugenol at concentration210-1M and to 34.63 µ A /cm2 in concentration 210-1M of eugenol in pH 2 medium. The results showed that eugenol revealed a good corrosion inhibitor. The inhibition efficiency depended by the concentrations of eugenol and pH medium.
Table 1. Summary of electrochemical parameters for titanium grade2 in medium saliva containing bacteria at different Concentration of eugeno
|
Concentration of eugenol |
I corr (µA/cm2) |
Rp (ohm cm2) |
E (i=0) mv |
E% |
|
0 M |
53.30 |
1060 |
-1111.8 |
|
|
10-1M |
33.29 |
2170 |
-1278.7 |
37.54 |
|
210-1M |
8.10 |
6120 |
-1236 |
84.80 |
Table 2. Summary of electrochemical parameters for titanium grade2 in medium saliva containing bacteria at different Concentration of eugenol
|
Concentration of eugenol |
I corr (µA/cm2) |
Rp (ohm cm2) |
E (i=0) mv |
E% |
|
0M |
111.99 |
111.99 |
-1493.5 |
|
|
10-1M |
74.05 |
988.41 |
-1336 |
33.87 |
|
210-1M |
34.63 |
2170 |
-1287.5 |
69.07 |
An impedance spectroscopy study was performed in order to confirm the results obtained with polarization tests.
EIS Measurements
The corrosion behaviour of titanium grade 2, in saliva medium containing bacteria at different pH, in the absence and presence of inhibitors, was also investigated by EIS after 30 minutes of immersion (Figure 3 and Figure 4). The locus of Nyquist plots is regarded as one part of semi circle. The same result as obtained with Kraljic in phosphoric acid and in saliva medium containing fluoride and eugenol [10-11].
Figure 3 and Figure 4 show the impedance diagrams recorded for titanium grade 2 in different medium to examine the concentration of eugenol effect. In case bacteria medium at pH=2 and pH=7 the impedance curve is in the form of a half-circle which can be attributed to electron transfer step. The diameter of circle increased with increasing the concentration of eugenol in the both medium; the corrosion resistance measurement of dental alloys has been studied by several authors [12-13].
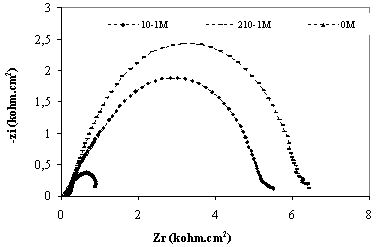
Figure 3. Electrochemical impedance spectroscopy for titanium grade 2 in saliva medium at PH=7 containing bacteria at different concentration of eugenol
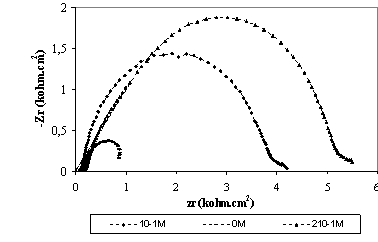
Figure 4. Electrochemical impedance spectroscopy for titanium grade 2 in saliva medium at PH=2 containing bacteria at different concentration of eugenol
The SEM micrographs (Figure 5) of titanium grade 2 after electrochemical analyses confirmed the electrochemical results They show a general change in the surface of titanium in the both medium, in the much more aggressive bacteria-acidified saliva medium the metal is characterized by localized pitting and adhesion of bacteria on the surface. Adhesion of microorganisms to the medical devices and these problems are widely studied in several works [14-16]. Microbial activity within biofilm formed on surface of metallic materials can affect the kinetics of cathodic or anodic reactions [17] and can also considerably modify the chemistry of any protective layer leading to either acceleration of corrosion [18-19]. However, the addition of eugenol in medium containing bacteria at pH 2 led the formation of film on the surface which increases the metal resistance and inhibits attached bacteria in the surface.
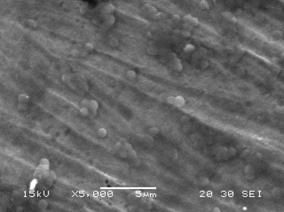
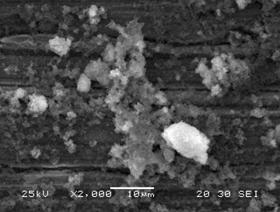
Figure 5. SEM of titanium after test electrochemical a) medium containing bacteria at pH2, b) medium containing bacteria at pH2+eugenol
Conclusions
Behaviour of titanium in a solution containing bacteria at different pH and eugenol were studied electrochemical properties, surface analyses by SEM and pH. These results are summarized as following:
· The corrosion resistance of titanium specimens solution containing bacteria at PH=7 decreased in comparison with that in a solution containing bacteria at pH=2. Adding eugenol to the solution resulted in preventing the corrosion of the titanium from the bacteria.
· The rate corrosion of titanium in medium saliva (pH 7) by adding eugenol was clearly lower than that in the solution containing bacteria at pH 7.
· From these results, the effects of eugenol are not only the protection of titanium from bacteria attack but also the suppression of dissolution of titanium ions via formation of the eugenol films. These effects evidently suppress the corrosion of titanium by bacteria.
References
1. Beech I.B., Corrosion of technical Materials in the Presence of Biofilms-Current Understanding and State-of-the-art Methods of Study, .Int Biodeterior Biodegradation 2004, 53, p. 177-183.
2. Beech I.B., Coutinho C.L.M, Biofilms on Corroding Materials. In Biofilms in Medicine; Industry and Environmental Biotechnology- characteristics; analysis and control. Edited by Lens P; Moran AP; Mahony T; Stoodly P; O’FlahertyV: IWA Publishing of Alliance House; 2003, p. 115-131.
3. Baker P.W., Ito K., Watanabe K., Marine Prosthecate Bacteria Ennoblement of Stainless Steel, Environ Microbial, 2003, 5, p. 925-932.
4. Kjellerup B.V., Olesen B.H., Nielsen J.L., Frolund B., Odum S., Nielsen P.H., Monitoring and Characterisation of Bacteria in Corroding District Heating Systems Using Fluorescence in Situ Hybridisation and Microautoradiography, Water Sci Technol, 2003, 47, p. 117-122.
5. Zlation T., Fang H.H.P., Ko B.C.B., Methanogen Population in a Marine Biofilm Corrosive to Mild Steel, Appl Microbiol Biotechnol, 2003, 63, p. 101-106.
6. Bregmann J.I., Corrosion Inhibitors, P. T. Macmillan, New York, 1963.
7. Hakermann N., Langmuir, 1987, 3, p. 922.
8. Nathan C.C., Organics Inhibitors, NACE, Houston, 1977.
9. Trabanelli G., Caraaiti V., Fontana M.G., Staehle R.W., (Eds.), Advances in Corrosion Science and Technology, 1, Plenum Press, NY, 1970, 147, 20.
10. Duic L.J.Z., Inhibition of Steel Corrosion by Polyaniline Coating, Corros. Sci., 2003, 45, p. 181-198.
11. Kinani L., Najih R., Chtaini A., Corrosion Inhibition of Titanium in Artificial Saliva Containing Fluoride, Leonardo Journal of Sciences, 2008, p. 1583-0233.
12. Al-Hity R.R., Kappert H.F., Viennot S., Dalard F., Grosgogeat B., Corrosion Resistance Measurements of Dental Alloys, are they Correlated?, 2007, 23(6), p. 679-687.
13. March P., Berthod P., Dervisevic B., Vallata A., Comportement électrochimique d’alliages et brasures dentaires utilisés en prothèse fixée dans une salive artificielle fusayama, P.B. Sci. Bull, 2010, 72(2), p. 1454-2331.
14. Hamadi F., Latrache H., Mliji E., Mallouki B., Mabrouki M., Ellouali M., Adhésion de Staphylococcus aureus au verre et au téflon, Revue de Microbiologie, 2009, 3(1), p. 1-16.
15. Kouider N., Hamadi F., Mallouki B., Bengoram J., Mabrouki M., Zekraoui M., Ellouali M., Latrache H., Effect of Stainless Steel Surface Roughness on Staphylococcus Aureus adhesion, International Journal of Pure and Applied Science, 2010, 4(1), p.1-7.
16. Mallouki B., Latrache H., Hamadi F., Bengourram J., El Mhammedi M. A., Chtaini A., Mabrouki M., Outzourhit A., Muller D., El Louali M., Staphylococcus aureus adhesion to steel surfaces in the presence of fucans, Microbiol. Hyg. Alim, 2010, 22(64), p. 28 -37.
17. Jones D.A., Amy P.S., A Thermodynamic Interpretation of Microbiologically Influenced Corrosion, Corrosion, 2002, 58, p.638-645.
18. Little B., Ray R., A Perspective on Corrosion Inhibition by Biofilm, Corrosion, 2002, 58, p. 424-428.
19. Ornek D., Wood T.K., Hsu C.H., Sun Z., Mansfeld F, Pitting Corrosion Control of Aluminium 2004 Using Protective Biofilms that Secrete Corrosion Inhibitors, Corrosion, 2002; 58, p. 761-767.