Effects of manganese on corrosion behaviour of welded Al-Fe-Si alloy in NaCl medium
Isiaka Oluwole OLADELE, Oluyemi Ojo DARAMOLA*, Lawrence Ojo SALIU
Metallurgical and Materials Engineering Department, Federal University of Technology, PMB 704, Akure, Ondo State, Nigeria
E-mails: wolesucess2000@yahoo.com; ojaythompsoms@yahoo.com*; lawrenceojo@yahoo.com
* Corresponding author, Phone: +2348034677039
Abstract
The effects of manganese on the corrosion behaviour of welded and un-weld as–cast Al-Fe-Si alloy has been studied. Alloys of varying percentage of manganese from 0.019-0.24 (0.019, 0.15, 0.16, 0.17, 0.24 wt. %) were sand cast into rectangular test sheets of dimension 300´20´5 mm. Each of the alloys were cut into two equal parts and welded together. The corrosion characteristics of the five categories of alloys (welded and un-weld) in 0.5M NaCl at room temperature over a period of 1464 hours were investigated by weight loss method. The results obtained showed that Al-Fe-Si samples (un-weld and welded) in 0.5 M NaCl medium displayed a very good resistant to corrosion according to the percentage of manganese added. It was observed that sample A1 and A2 with manganese percentage of 0.17Mn in NaCl medium displayed a very good resistance to corrosion while sample D1 and D2 with manganese percentage of 0.24Mn in the same medium has the lowest corrosion resistance. The result actually confirmed that the addition of manganese aided corrosion resistance but to a certain level, since the addition of 0.150-0.170Mn leads to progressive inhibiting but after this range the corrosion rate increases.
Welding; Corrosion; Manganese; Sand cast
Introduction
Aluminium and its alloys are characterized by a relatively low density (2.7g/cm3 as compared to 7.9g/cm3 for steel and 8.86g/cm3 for copper), high electrical and thermal conductivities and resistance to corrosion in some common environments such as atmosphere, water, salty water [1,2,3]. This qualities makes aluminium alloys one of the most used nonferrous alloys used in the production of automotive components, construction materials, container and packaging, marine, aviation, aerospace and electrical industries [4]. The good properties and low cost of aluminium alloys have resulted in such increased use that in 1990, aluminium was the second most widely used metal, second only to steel [5,6].
Based on these good mechanical properties, the alloy can be forged, stamped, extruded and sand cast [7]. Due to the numerous applications of aluminium in a variety of corrosive environments, different methods / processes have been investigated with the aim of increasing the corrosion resistance of aluminium alloys [1].
Aluminium and its alloys are considered to be highly corrosion resistant under majority of service conditions [2]. The various grades of pure aluminium are most resistant, followed closely by the Al-Mg and Al-Mn alloys. Next in order is Al-Mg-Si and then Al-Si alloy. The alloy containing copper are the least resistant to corrosion; but this can be improved by coating each side of the copper containing alloy with a thin layer of high purity aluminium, thus gaining a three – ply metal, i.e. Alclad. This cladding acts as a mechanical shield and also protects by sacrificial corrosion at cut edges [8]; when aluminium surface are exposed to the atmosphere, a thin invisible oxide (Al2O3) skin forms, which protects the metal from corrosion in many environments.
Aluminium base alloys also have high thermal conductivity and low coefficient of linear expansion values and based on these two properties, they can be forged to the desired shapes at elevated temperatures and then solution treated and aged hardened to obtain desired microstructures and excellent mechanical properties [9, 10].
Al-Si-Fe, a ternary alloy provides good combination of cost, strength, corrosion resistance, together with high fluidity freedom from hot shortness [11]. The compositional specification of these alloys rests mainly on the iron, silicon and manganese contents while addition of manganese alone improves the toughness of the alloy [12]. Iron also increases strength and hardness, reduces tendency to hot cracking while silicon improves the fluidity of the molten metal as well as its castabilty and mechanical properties [13].
The types of corrosion that affect Al- alloys are galvanic, stress corrosion cracking, intergranular, crevice [14].
The special objective of the research work is to determine the individual and simultaneous effects of concentration of manganese and time of exposure on the corrosion resistance of Al-Fe-Si alloys in 0.5M NaCl at 28°C for 1646 hours in the as-cast and welded conditions.
Material and method
The materials used for this research work for the production of Al-Fe-Si-Mn (0.2%), Al-Fe-Si-Mn (0.15Mn), Al-Fe-Si-Mn (0.16Mn) and Al-Fe-Si-Mn (0.17Mn) alloy systems includes high purity aluminium obtained from Nigalex Nigeria Limited, Lagos and ferroalloys (FeSi and FeMn), sodium chloride (NaCl), etchants, baker, thread (for suspending the coupons in corrosive media), measuring cylinder, silica sand, bentonite, moulding boxes.
The following equipment was used; Muffle electrical resistance furnace, polishing machine, metallurgical microscope, and digital weighing balance.
The different compositions of alloys were produced in which the amount of Mn in the alloy were varied. Each composition was melted separately in alumina crucible. The melting was done using a muffle resistance furnace that was allowed to heat to 750°C before the crucible was returned into the furnace for further 30 minutes during which the furnace temperature was raised to 800°C for superheat to occur. The molten metal was stirred thoroughly before pouring into the prepared sand mould for casting. The cast samples were machined into corrosion test specimens and designated as A1, B1, C1, D1, and E1. Some of the cast samples were also cut into two equal parts, welded together, machined into corrosion test specimens and designated as A2, B2, C2, D2, and E2.
Table 1: Chemical composition of as-cast aluminum alloys.
|
Samples |
Al |
Si |
Fe |
Mn |
Mg |
Zn |
Cr |
Ni |
Cu |
|
A1 |
98.9 |
0.170 |
0.670 |
0.170 |
0.0016 |
0.0100 |
0.0049 |
0.0066 |
0.034 |
|
B1 |
99.3 |
0.080 |
0.402 |
0.150 |
0.0006 |
0.0028 |
0.0044 |
0.0017 |
0.021 |
|
C1 |
99.0 |
0.119 |
0.610 |
0.160 |
0.0011 |
0.0059 |
0.0031 |
0.0036 |
0.028 |
|
D1 |
99.0 |
0.118 |
0.510 |
0.214 |
0.0007 |
0.0470 |
0.0038 |
0.0032 |
0.0028 |
|
E1(control) |
99.5 |
0.095 |
0.311 |
0.019 |
0.0014 |
0.0272 |
0.0049 |
0.0066 |
0.0073 |
Corrosion test
A total of 10 corrosion coupons were used for the research, 5 each for the as – cast and welded specimens respectively. Before immersion, the coupons were cleansed, weighed and stored in the desiccators. Sets of same concentrations of 0.5M NaCl and 0.5M H2SO4 solutions were prepared (one for the as-cast and the other for the welded parts). The cleaned coupons were suspended in NaCl and H2SO4 solutions with the aid of threads; the weight losses of the coupons were taken at intervals of two days over a period of sixty one days. After removal from the solution, the surface of each coupon was scrubbed with brush in distilled water, rinsed in ethanol and then dried in air and weighed. The corrosion rate was determined in Mils per year (mpy). The corrosion rate conversion between mpy and the equivalent in metric unit mm/y (milimeter/year) was made using:
1 mpy = 0.0254 mm/y = 25.4 microm/y
The corrosion rate from metal loss was calculated using:
mm/y = 87.6 ∙ (W/DAT)
The corrosion rate was:
mpy = 534 W/DAT
where; W= weight loss in milligrams; D = density of materials in g/cm3; A = Cross sectional area in cm2; T = Exposure time in hours.
Metallographic examination
Metallographic specimens were cut from the as-cast alloys and the welded portion of the alloys. The specimens were then mounted in Bakelite and mechanically ground progressively on grades of SiC impregnated emery paper (80-600 grits) sizes using water as the coolant. The ground specimens were then polished using one –micron size alumina polishing powder suspended in distilled water. Following the polishing operation, etching of the polished specimens was done using Keller’s reagent and the structures obtained were then photographically recorded using a metallurgical microscope.
Results and discussion
Effect of manganese on the corrosion behaviour of as-cast aluminium alloy and welded samples in 0.5M NaCl medium
The results of the corrosion rate of the as-cast aluminium alloys and the welded specimens are shown in Figures 1-2, it can be seen that, the corrosion rate decreases for both un-weld and welded Al-Fe-Si alloys as the amount of Mn was increased up to 0.17% with the unweld alloys possessing better corrosion resistance than the welded alloys. The reason for the lower corrosion rate of un-weld and welded alloys may be due to the precipitation of the intermetallic compounds and the amount of intermetallic precipitated increase with increase in Mn additions. It could be observed in Figure 1 and Figure 2 that addition of Mn to Al-Fe-Si alloy in the as cast and welded samples made the alloys to have better corrosion resistance due to the formation of hard and passive phases that acted as strong protective barriers to corrosion. Specifically, Figures 1and 2 shows that after 1464hrs. Exposure, 0.17% Mn alloy have the best corrosion resistance for the unweld sample and welded sample respectively. The corrosion rates decreases with increase in exposure time for all level of conditions.
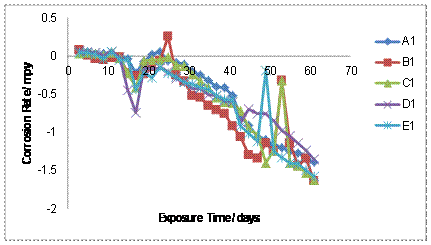
Figure 1. Corrosion rate of un-weld samples A1, B1, C1, D1, E1 in 0.5M NaCl
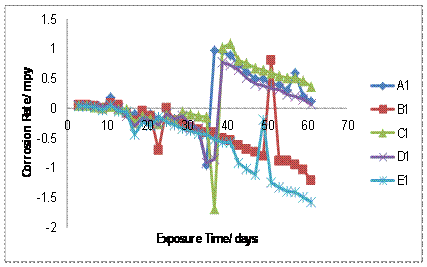
Figure 2. Corrosion rate (mpy) of welded samples A2, B2, C2, D2, E2 in 0.5M NaCl
Microstructure
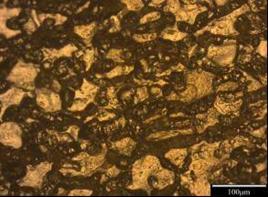
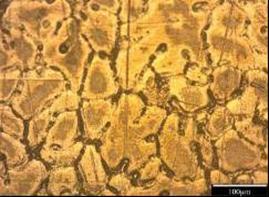
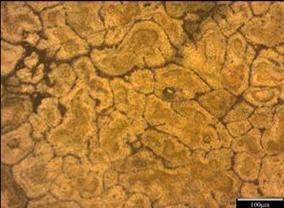
As shown in Figure 3-12, ten different microstructures have been achieved. Manganese as a neutralizer is often added to Al-Fe-Si alloy to reduce embrittlement and alter the shape from sharp needles to a more compact type which is more or less equiaxed and tending to form as dendrites with α-phase morphology. According to the present investigation as shown in Figure 3-13, the iron –rich needles Al-Fe-Si are always present and coexisting l-with α- phase Al-(Fe, Mn)-Si as long as iron and manganese are present.
|
Figure 3. Photomicrograph of un-weld sample A1 in 0.5M NaCl (x100) |
Figure 4. Photomicrograph of un-weld sample B1 in 0.5M NaCl (x100) |
|
Figure 5. Photomicrograph of un-weld sample C1 in 0.5M NaCl (x100) |
Figure 6. Photomicrograph of un-weld aluminum sample D1 in 0.5M NaCl (x100) |
|
Figure 7. Photomicrograph of un-weld aluminum sample E1 in 0.5M NaCl (x100) |
|
The morphology of the α- phase is altered to a more co-existing star-like and polyhedral type as manganese level is increased as seen in Figure 3-7, volume fraction, coarser and more complex intermetallic are likely to develop and the size of each of them seem to be a function of the cooling rate and of the Mn and Fe level in the melt as they might precipitate as post dendrite and/or as pre-or post- eutectic [13]
Generally speaking, it seems that when adding Mn, the alloys display shorter iron - rich needles which might be due to less amount of iron available in the melt since the formation of α-phase and other intermetallic are promoted as well. At higher Mn level, sludge formation might be taking place due to an improper melt handling process and high level of Mn exceeding the recommended sludge factor [14, 15]. This can be seen in Figure 7 when the manganese content is 0.214 where the Fe, Mn, and Cr might have precipitated as sludge which should absolutely be avoided since its presence might be deleterious to the overall properties of the alloy.
|
Figure 8. Photomicrograph of welded sample A2 in 0.5M NaCl (100) |
Figure 9. Photomicrograph of welded sample B2 in 0.5 NaCl (x100) |
|
Figure 10. Photomicrograph of welded sample C2 in 0.5M NaCl (x100) |
Figure 11. Photomicrograph of welded sample D2 in 0.5M NaCl |
|
Figure 12. Photomicrograph of welded sample E2 in 0.5M NaCl (x100) |
|
The corrosion rate decreases for both un-weld and welded alloys as the amount of Mn was increased up to 0.17% with the un-weld alloy possessing better corrosion resistance than the welded samples
Conclusions
This work has shown clearly that the aluminum alloy developed could not be affected by corrosion in NaCl medium in the welded and un-weld conditions due to the protective film formed on the surface of the samples.
Sample A was observed to have the best corrosion resistance with manganese percentage of 0.170Mn which is observed to be the best manganese percentage that should be added to Al-Fe-Si alloy in order to be able to withstand corrosion in NaCl medium to some extent.
Manganese percentage addition should not be more than (0.150Mn--0.170Mn) in order for Aluminum to have a very good corrosion resistance.
Conclusively sample A1, B1, C1 resist corrosion more than the other samples, but sample A1 was observed to be the best
References
1. Ravikumar E; Arunkumar N. and Sunnapa G.S; Characterization of mechanical properties of aluminium (AA 6061-T6) by friction welding, 3rd International Conference on mechanical, automotive and materials engineering (ICMAME), April 29 – 30, 2013, Singapore.
2. Madugu I.A. and Abdulwahab M; .The mechanical properties of as-cast Al-Si-Fe alloy, Nigerian journal of research and production, 2007, 10; p. 72-80.
3. Polmear I.J., and Edward A., Light alloys (Metallurgy of light metals), Second edition, 1993; p. 124-167.
4. Barrow, G.M; Physical chemistry of metals, 4th Edition, McGraw-Hill Book Company, New York. 1987.
5. Adamson, W.A; Physical chemistry of surfaces, 5th Edition, John Wiley and Sons, Inc., New York. 1997.
1. Polmear I.J., and Edward A., Light alloys (Metallurgy of light metals), Third edition, 1995, p. 134-160.
6. Sindo K., Welding metallurgy, 2nd edition, New York, John Wiley & Sons, 2003
7. Degarmo E.P, Corrosion resistance of aluminum, financial evaluation of small scale corrosion projects in Ghana – A case study, MSc Thesis, University of material studies and Technology, Tarkwa, 2003, p.92.
8. Abdulwahab M; Studies of the mechanical properties of age– hardened Al-Si-Fe-Mn alloy, Australian journal of basic and applied sciences, 2008, 2(4), p.839-843.
9. Oladele I.O. and Omotoyinbo J.A., Effect of welding current and voltage on the mechanical properties of wrought (6063) aluminium alloy, Material research journal, 2010, 13(2), p. 125-128.
10. Ovat F.A; Asuquo L.O. and Anyandi A.J, Microstructural effects of electrodes types on the mechanical behaviour of welded steel joints, Research journal in engineering and applied sciences, 2012, 1(3) p.171-176.
11. Subbaiah K; Geetha M; Grovindarajan and Rao S.R.K. Friction stir welded joints of cast scandium added aluminium- magnesium alloy, International journal of advanced engineering technology, 2011, 2(3), p.10-13.
12. Lancaster J.F., Metallurgy of welding, 5th edition, London, Chapman and Hall Publisher, 1994.
13. Scully, J.C; The fundamental of corrosion, Maxwell Macmillan Perganman Publishing Corporation, Oxford. 1990.
14. International Standards for Materials – ASTM, Trends welding research in the United States, Ohio Materials Park, 2003.