Characterization of some clay deposits in South West Nigeria
* Fatai Olufemi ARAMIDE1, 2, Kenneth Kanayo ALANEME 1,2, Peter Apata OLUBAMBI 3 and Joseph Olatunde BORODE 1,2
1Department of Metallurgical and Materials Engineering, Federal University of Technology, P.M.B. 704, Akure, Nigeria
2African Materials Science and Engineering Network, (AMSEN) a Subsidiary of Regional Initiative for Science Education (RISE)
3Applied Microscopy and Triboelectrochemical Research Laboratory, Department of Chemical and Metallurgical Engineering, Tshwane University of Technology, Pretoria, South Africa
E-mails: foaramide@futa.edu.ng, fat2003net@gmail.com
* Corresponding author, Phone: +2348038509288
Abstract
Clay minerals are the most important industrial minerals whose application is dependent on its structure and chemical composition. Mineralogical, chemical compositions, phase constitutions, and microstructural morphology of certain clay minerals from three different deposits in south western Nigeria were investigated using state-of-the-art equipment. These were done with the intention of determining the appropriate application for the clay minerals. It was observed that the major phases in the clay samples from the three different deposits are kaolinite, microcline, muscovite/illite, plagioclase/albite and quartz. These phases were observed in varied percentages. It was concluded that sample A (Ifon clay) which contains very low kaolinite (5.63%); could not use for making high temperature caliber refractories. But due to its high content of feldspar, it could be processed for the production of feldspar for glass and iron making industries. Sample B is considered to be appropriate for the production the refractory composite due to its most appropriate content of both kaolinite (23.74% kaolinite) and feldspars (26.12% microcline and 11.28% plagioclase/albite) which is necessary for producing mullite fibers in ceramic matrix at a temperature of around 1400oC. Sample C (Iseyin clay), which contains very low feldspars (3.00% microcline and 3.08% plagioclase/albite) and high content of kaolinite was considered suitable for further processing for making high temperature caliber refractories.
Keywords
Characterization; Clay minerals; Feldspar; Kaolinite; Raw materials; Mineralogical composition; Industrial application
Introduction
Clay minerals are the most important industrial minerals. Millions of tons are utilized yearly in various applications. These applications include uses in geology, the process industries, agriculture, environmental remediation and construction.
The reason for utilization of certain clay minerals in specific application is that the physical and chemical properties of a particular clay mineral are dependent on its structure and composition. The structure and composition of kaolins, smectites, and palygorskite and sepiolite are very different even though they each have octahedral and tetrahedral sheets as their basic building blocks. However, the arrangement and composition of these octahedral and tetrahedral sheets account for major and minor differences in the physical and chemical properties of kaolin, smectites and palygorskite [1].
Clays have received considerable attention especially as potential adsorbents for environmental research. Many researchers around the world, have beamed their search lights on the phase developments that occurred by sintering clay in the presence of some oxides [2, 3, 4].
Deposits of clay raw material are widely distributed in Nigeria [5-12]. In order to determine the profitability of utilizing clay from a particular deposit for any application, it is of paramount importance to examine the microstructural morphology, determine the mineralogical composition and analyse the various available phases in such clay deposit. This work is aimed at characterization of clays from three different deposits in South West Nigeria. The deposits under investigation include; Ifon clay (Sample A), Ipetumodu clay (Sample B) and Iseyin clay (Sample C).
Ifon is a town in Ondo State, Nigeria. Its geographical coordinates are 6°55' 0" North, 5°46' 0" East. It is the headquarters and seat of government of Osẹ Local Government Area. Ifon is a junction town with two axes to Edo State and one to the rest of Ondo State. It lies at about the mid-point on the Federal Highway that connects Akurẹ and Benin City. It has a population of over Forty Six Thousand (46,000) in the 1991 census.
Ipetumodu is a town in Osun State, in the southwestern part of Nigeria. The size of the town is 64 km2 (25 sq/mi). The town is the headquarters of Ife North local government Area. It is located at an elevation of 227 meters above sea level and its population amounts to 164,172. Its coordinates are 7°31'0" N and 4°27'0" E.
Iseyin is an ancient town in Oyo State, Nigeria. It is located on Latitude 7° 59' 38" N and Longitude 3° 35' 51" E. It has a population range of between 100,000 and 250,000. Location of Iseyin in The Times Comprehensive Atlas of the World is plate 86 C8.
The aim of this investigation is to predict accurate applications for each of the clay.
Material and method
Clay samples used in this study were sourced from selected locations in the south western part of Nigeria, namely; Ifon in Ondo State, Ipetumodu in Osun State and Iseyin in Oyo State. The clay samples were collected according ASTM D4220M – 14.
The clay samples were first soaked in water for three days to dissolve the deleterious materials in them and at the same time to form slurry. The slurries were then sieved to remove deleterious materials and other foreign substances. The sieved slurries were then allowed to settle down for three days after which the clear floating water was decanted. The dispersed fine clays in water (clay slurries) were then poured into plaster of Paris (P.O.P) moulds and left undisturbed for three days in order to allow the remaining water present to drain out completely.
The resulting plastic clay masses were sun dried and subsequently dried in a laboratory oven at 110°C for 24 hours. The resulting dried clay samples were crushed and milled in a Rawwley Sussex grinder to an average particle size of 300µm. From this representative samples were collected and sent for various analysis.
Qualitative and quantitative XRD
The samples were prepared for XRD analysis using a back loading preparation method [13]. They were analysed using a PANalytical X’Pert Pro powder diffractometer with X’Celerator detector and variable divergence- and receiving slits with Fe filtered Co-Kα radiation. The phases were identified using X’Pert Highscore plus software. The receiving slit was placed at 0.040°. The counting area was from 5 to 70° on a 2θ scale. The count time was 1.5 s. The temperature-scanned XRD data were obtained using an Anton Paar HTK 16 heating chamber with Pt heating strip Graphical representations of the qualitative result follow below.
The relative phase amounts (weight %) was estimated using the Rietveld method (Autoquan Program) as reported by Young et al [14]. Amorphous phases, if present were not taken into consideration in the quantification. The results obtained are presented in Figures 1, 2, 3 and Table 2.
Scanning electron microscopy
Morphology and microanalysis of the clay and composite samples were determined using ultra-high resolution field emission scanning electron microscope (UHR-FEGSEM) equipped with energy dispersive spectroscopy (EDS). The pulverized clay samples were previously gold coated. The samples were studied using ultra-high resolution field emission scanning electron microscope (UHR-FEGSEM) equipped with energy dispersive spectroscopy (EDS). Particle images were obtained with a secondary electron detector. The SEM/EDS micrographs of the powder clay samples are shown in Figures 4 a, b and c.
Chemical analysis
(a) X-Ray Fluorescence (XRF)
The major elements were determined by X-ray fluorescence with an ARL® 9800 XP spectrometer. The pulverized clay samples were mixed with lithium tetraborate for chemical analysis. The ignition loss was measured by calcinations at 1000 °C. The XRF result is shown in Table 1
(b) Atomic Absorption Spectroscopy (AAS)
The chemical analysis of the clay samples was also carried out using atomic absorption spectrophotometry (AAS) method with Spectra AA 220 FS Machine. The percentage compositions of the various constituents were then recorded in Table 3.
Results and discussion
Table 1, 2 and 3 respectively shows XRF Semi-quantitative analysis of the elements of raw clay samples, XRD results of the raw clay samples showing the quantity of different phases present (weight %), and Atomic Absorption Spectroscopy Result of raw Clay Samples (weight %). Figures 1, 2 and 3 respectively shows X-ray diffraction pattern of sample A: Identified major mineral phases includes silica, iron oxide, magnesite, and alumina, X-ray diffraction pattern of Sample B Identified major mineral phases includes silica, alumina, hematite and titania, X-ray diffraction pattern of Sample C Identified major mineral phases includes; silica, alumina, hematite and titania.
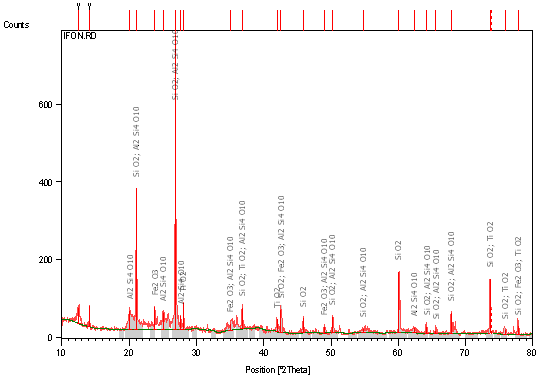
Figure 1. X-Ray diffraction pattern (phase analysis) of sample A
From Table 2 the different phases that exist in the raw clay samples are clearly shown; it is seen that raw samples A and B feldspar’s composition are higher than any other phases present in the clay samples, while sample C is observed to contain very low feldspar in comparison with samples A and B. According to Brown et al. [15] feldspars are the most abundant mineral group found in Earth’s crust. Albite (NaAlSi3O8) anchors the two main feldspar compositional series: the alkali feldspars (Na, K) AlSi3O8 and the plagioclase series (Na, Ca)Al(Si, Al) Si2O8.
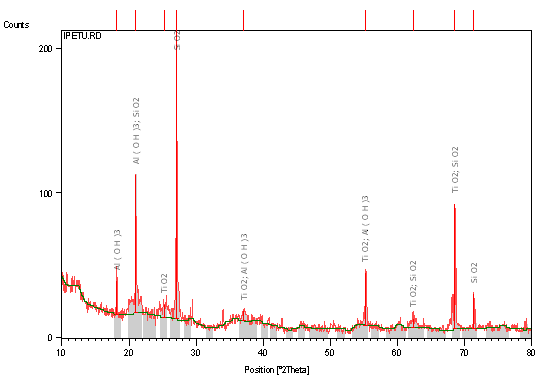
Figure 2. X-Ray diffraction pattern (phase analysis) of sample B
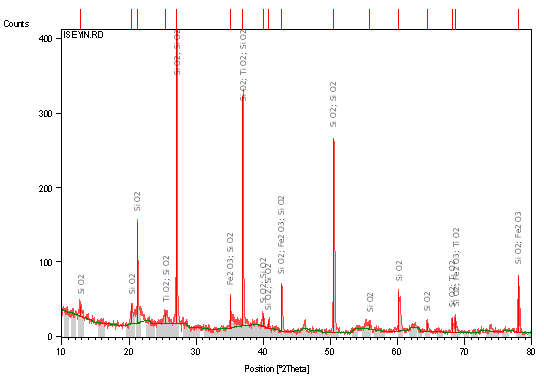
Figure 3. X-Ray diffraction pattern (phase analysis) of sample C
Albite has triclinic symmetry, which is common for the "low temperature" alkali-feldspar and albite-rich plagioclase with ordered aluminium and silicon in the tetrahedral sites. This could account for the presence of potassium, sodium and calcium oxides in the clay deposits as show in Tables 1 and 2. The presence of Magnesium oxide in the clay samples suggests the presence of a talc phase, which could also accounts partly for the presence of the iron oxide [16].
Table 1. XRF analysis of the elements of raw clay samples (weight %)
|
Phases |
A |
B |
C |
|
Al2O3 |
22.42 |
25.03 |
22.729 |
|
SiO2 |
63.35 |
57.482 |
62.292 |
|
Fe2O3 |
6.109 |
9.226 |
7.266 |
|
K2O |
2.878 |
1.259 |
1.36 |
|
MgO |
1.351 |
0.91 |
0.985 |
|
CaO |
0.689 |
0.763 |
0.541 |
|
Na2O |
0.789 |
0.372 |
0.259 |
|
TiO2 |
0.923 |
1.512 |
1.046 |
|
Zr |
0.045 |
0.103 |
0.056 |
|
Total |
99.1 |
97.1 |
97.1 |
Table 2. Chemical composition of raw clay samples (%)
|
Samples |
SiO2 |
Al2O3 |
Fe2O3 |
TiO2 |
CaO |
MgO |
Na2O |
K2O |
LOI* |
|
A |
56.77 |
27.46 |
1.32 |
1.96 |
0.18 |
0.14 |
0.04 |
1.62 |
10.27 |
|
B |
54.82 |
25.90 |
1.44 |
2.08 |
0.12 |
0.15 |
0.2 |
1.57 |
11.64 |
|
C |
59.42 |
35.88 |
2.42 |
1.40 |
0.17 |
0.15 |
0.04 |
0.09 |
11.66 |
*LOI = Loss on Ignition
The presence of iron in the clay samples is clearly observed although in various amounts; this has been supported by various researchers as a rule rather than exception [17-28]. From this it can be said that samples A and B except with the addition of some additive that will improve on their refractory properties, cannot be used to produce good refractory materials for high temperature applications because of their high content of feldspars which favor liquid phase formation and densification at low temperature [29-34].
Moreover, feldspars are important raw materials for ceramic and glass industries and chemical compositions; especially alkali contents determine their quality and price [35]. The most part of feldspars used in the industry are potassium-sodium feldspars. The most important of them are microcline and a microcline-perthite (microcline with plagioclase interpositions). Industrial application of feldspars is based on their ability to fuse at relatively low temperatures generating glass.
Feldspar with other compounds (Kaolin, quartz, etc.) generates porcelain. Therefore the basic fields of application of feldspar are ceramics and glass industries, where it is one of the major components in ceramic, glass, glaze and enamel mixtures.
Table 3. XRD Result of the raw clay samples showing the quantity of different phases Present
|
Phases |
A (weight %) |
B (weight %) |
C (weight %) |
|
Kaolinite |
5.63 |
23.74 |
39.71 |
|
Microcline |
30.90 |
26.12 |
3.00 |
|
Muscovite/Illite |
3.81 |
15.02 |
14.66 |
|
Plagioclase/ Albite |
18.22 |
11.28 |
3.08 |
|
Quartz |
41.42 |
23.84 |
39.55 |
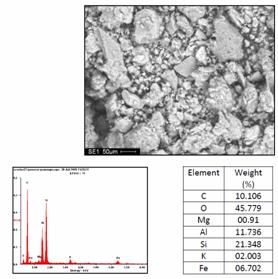
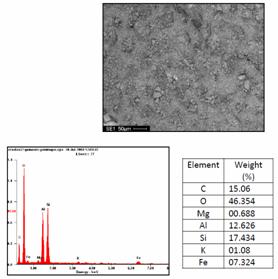
(a) (b)
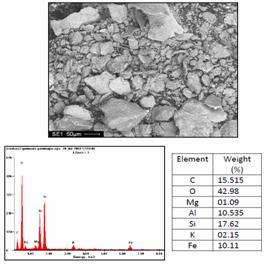
(c)
Figure 4. Typical SEM/EDS micrographs of (a) Sample A, (b) Sample B (c) Sample C: showing the morphology of the mineral and its chemical composition
From Table 3, it is observed that sample A (Ifon clay) contains the least amount of kaolinite (5.63%) and muscovite/illite (3.81%) compare to the other two clay samples. This made it (sample A) a candidate for further processing for the production of feldspar. Sample B contains comparatively high contents of kaolinite and feldspar hence it is a candidate for the production of mullite fibber reinforced ceramic composite (since it is the kaolinite that will form mullite when fired above 1400°C in the presence of enough feldspar).
Feldspar is necessary for the production of liquid phase during sintering which accelerates reactions and contributes to the formation of high-temperature crystalline compounds and densification of ceramics [36]. Sample C has low content of feldspar and comparatively high content of kaolinite; this makes it suitable for the production of high temperature caliber refractories.
Conclusions
From the generated and analyzed data in the research work, it could be concluded that;
· The major identified phases in the clay samples from the three different deposits are kaolinite, microcline, muscovite/illite, plagioclase/albite and quartz;
· Sample A (Ifon clay) contains very low kaolinite (5.63%); could not use for making high temperature caliber refractories. But due to its high content of feldspar, it could be processed for the production of feldspar for glass and iron making industries
· Sample B is considered to be appropriate for the production the refractory composite due to its most appropriate content of both kaolinite (23.74% kaolinite) and feldspars (26.12% microcline and 11.28% plagioclase/albite) which is necessary for producing mullite fibers in ceramic matrix at a temperature of around 1400°C
· Sample C (Iseyin clay) contains very low feldspars (3.00% microcline and 3.08% plagioclase/albite) and high content of kaolinite. This makes it suitable for further process for making high temperature caliber refractories.
Acknowledgements
The authors wish to acknowledge the following organizations for their support. They are Regional Initiative in Science Education (RISE), Science Initiative Group (SIG) and African Materials Science and Engineering Network (AMSEN).
References
1. Murray H.H., Applied clay mineralogy today and tomorrow, Clay minerals, 1999, 34, p. 39-49.
2. Kong L.B., Gan Y.B., J. Ma J., Zhang T.S., Boey F., Zhang R.F., Mullite phase formation and reaction sequences with the presence of pentoxides, Journal of alloys and compound, 2003, 351, p. 264–272.
3. Biswajoy B., Sukhen D., Alakananda B., Ruma B., Papiya N., Mullite phase enhancement in Indian kaolins by addition of vanadium pentoxide, Applied clay science, 2010, 47, p.409- 413.
4. Wan P.C., Yu W., Xiao X.W., Jie W., Helen L.W.C., Water-induced dc and ac degradation in TiO2-based ceramic capacitors, Materials chemistry and physics, 2003 82, p.520-524.
5. Osarenmwinda J.A., Chukwuemeka P., Performance evaluation of refractory bricks produced from locally sourced clay materials, J. Appl. sci. environ. manage, 2014, 18(2), p.151-157.
6. Aye E. A., Oyetunji A., Metallurgical analysis of Ugunoda clay deposit, Nigeria for use as a refractory, International journal of science and advanced technology, 2013, 3(10), p.25-29.
7. Fasuba O.A., Egunlae O. and Jimoh B., Metallurgical analysis of Orin-Ekiti alumina clay deposit for use as a refractory, Journal of engineering technology and industrial applications, 2001, 1(4), p. 67-71.
8. Omowunmi O.J., Characterisation of some Nigerian clays as refractory materials for furnace lining, Nigerian journal of engineering management, 2001, 2(3), 1-4.
9. Irabor P.S.A., Physical and chemical investigation on some Nigerian kaolinite clays for use in the ceramics and allied industries, Nigerian journal of engineering research and development, 2002, 1(1), p.54-59.
10. Gbadebo A.M., Evaluation of engineering and industrial potentials of tidal flat clays in parts of niger delter, Nigeria. Nigerian journal of engineering research and development, 2002, 1(3), p.20-27.
11. Igbokwe P.K., and Ogbuagu J.O., Effects of process parameters on the extraction of alumina from indigenous kaolinitic clay deposit, Nigerian journal of engineering research and development, 2003, 2(2), p.23-26.
12. Odo J.U, and Nwajagu C.O., Possible application of Eha-Alumona clay deposit in the production of refractories and ceramic ware. Proceedings of the Nigerian materials congress and meeting of the Nigerian Materials Research Society at Conference Hall, Engineering materials development institute, Akure Nov. 12-14, 2003, p.109-111.
13. Kleeberg R., Monecke T., Hillier S., Preferred orientation of mineral grains in sample mounts for quantitative XRD measurements: How random are powder samples?, Clays and clay minerals, 2008, 56 (4), p.404-415.
14. Young R.A, Sakthivel A., Moss T.S., Paiva-Santos C.O., Rietveld analysis of X-ray and neutron powder diffraction patterns, School of physics, Georgia institute of technology, Atlanta, U.S.A. 1994.
15. Brown J.M., Abramson E.H., Angel R.J., Triclinic elastic constants for low albite, Phys and chem. of minerals, 2006, 33, p.256-265.
16. Marcos C., Rodríguez I., Expansion behaviour of commercial vermiculites at 1000°C, Applied clay science, 2010, 48, p.492–498.
17. Stucki J. W., Goodman B.A., Schwertmann U., Reidel D., Dordrecht, The Netherlands, 1988, p.625-675.
18. Badraoui M., Bloom P.R., Iron-rich high-charge beidellite in vertisols and mollisols of the High Chaouia region of Morocco, Soil Science Society of America Journal, 1990, 54, p.267-274.
19. Boiabid R., Badraoui M., Bloom P.R., Potassium fixation and charge characteristics of soil clays, Soil science society of america journal, 1991, 55, p.1493–1498.
20. Aoki S., Kohyama N., Vertical change in clay mineral composition and chemical characteristics of smectite in sediment cores from the southern part of the Central Pacific Basin, Marine geology, 1991, 98, p.41–49.
21. Badaut D., Decarreau A., Besson G., Ferripyrophyllite and related Fe3+-rich 2:1 clays in recent deposits of Atlantis II Deep, Red Sea, Clay minerals, 1992, 27, p.227–244.
22. Decarreau A., Grauby O., Petit S., The actual distribution of octahedral cations in 2:1 clay minerals: results from clay synthesis, Applied clay science, 1992, 7, 147-167.
23. Martin F., Petit M., Decarreau A., Grauby O., Hazemann J.L. and Noack Y., Experimental study of Si-Ge tetrahedral solid solution in Ni-Co-Mg talcs, Thin solid films, 1992, 222, p.189–195.
24. Petit S., Robert J.L., Decarreau A., Besson G., Grauby O. and Martin F., Contribution of spectroscopic methods to 2/1 clay characterization, Bulletin des centres de recherches exploration-production Elf Aquitaine, 1995, 19, p.119–147.
25. Grauby O., Petit S., Decarreau A. and Baronnet A. Nontronite-saponite series: an experimental approach, European journal of mineralogy, 1994, 4, p.99–112.
26. Grauby O., Petit S., Decarreau A. and Baronnet A., The beidellite-saponite series: an experimental approach, European journal of mineralogy, 1993, 5, p.623–635.
27. Martin F., Petit S., Decarreau A., Ildefonse P., Grauby O., Beziat D., Parseval P.D. and Noack Y., Ga/Al substitutions in synthetic kaolinites and smectites, Clay minerals, 1998, 33, p.231–241.
28. Giresse P. and Wiewiora A., Stratigraphic condensed deposition and diagenetic evolution of green clay minerals in deep water sediments on the Ivory Coast-Ghana Ridge, Marine geology, 2001, 179, p.51–70.
29. Ergul S., Akyildiz M., Karamanov A., Ceramic material from basaltic tuffs, Industrial ceramics, 2007, 27(2), p.89-94.
30. Aramide F.O., Effect of firing temperature on mechanical properties of fired masonry bricks produced from Ipetumodu clay, Leonardo journal of sciences, 2012, 21(2), p.70-82.
31. Reed J.S., Principles of ceramic processing, J. Wiley and Sons, 1995 New York.
32. Carty W.M., Senapati U., Porcelain - raw materials, Processing, phase evolution, and mechanical behavior, J. am. ceram. soc, 1998, 81(1), 3-20.
33. Manfredini T., Pellacani G., Romagnoli M., Pennisi L., Porcelainized stoneware tile, Am. ceram. soc. bull., 1995, 74(5), p.76-79.
34. Aramide F.O., Production and characterization of porous insulating fired bricks from Ifon clay with varied sawdust admixture, Journal of minerals and materials characterization and engineering, 2012, 11, p.970-975.
35. Bayraktar I. and Çakır U., Quality feldspar production at Çine Akınaden, Industrial minerals, 2002, p.56-59.
36. Sedmale G., Sperberga I., Sedmalis U., Valancius Z., Formation of high-temperature crystalline phases in ceramics from Illite Clay and Dolomite, J. eur. ceram. Soc., 2006, 26(15), p.3351-3355.