Kinetic modelling of the demineralization of shrimp exoskeleton using citric acid
Alewo Opuada AMEH1*, Muhammed Tijani ISA1, David ABUTU2, Alibaba DANLAMI1
1 Department of Chemical Engineering, Ahmadu Bello University, Zaria
2 Department of Chemical Sciences, Federal University Wukari, Taraba State, Nigeria
E-mails: alewooameh@yahoo.com; mtisaz@yahoo.com; abutleric@yahoo.com; alibaba_24000@yahoo.com.
* Corresponding author, Phone: +2348035600744
Abstract
Citric acid was used in the demineralization of shrimp exoskeleton and the kinetics of the demineralization process was studied. Kinetic data was obtained by demineralisation using five acid concentrations (0.1, 0.2, 0.3, 0.4 and 0.5M). The obtained kinetic data were fitted to the shrinking core model for fluid particle reactions. The concentration of calcium was found to decrease with time. For all acid concentrations considered, the best predictive mechanism for the demineralization process was determined to be Ash Layer Diffusion Control Mechanism. This was indicated by the high R2 values obtained (0.965) with 150% excess of citric acid.
Key words
Calcium; Citric acid; Demineralization; Shrimp; Shrinking core model
Introduction
Food products such as carapace and head of the shrimp, crab and krill originated from marine bio wastes is considered to be a rich source of protein, calcium carbonate and chitin [1]. Chitin is a natural polysaccharide composed from N-acetyl-D-glucosamine units and is the second most plentiful biopolymer after cellulose [2]. It is biodegradable, biocompatible and nontoxic; therefore, chitin and its deacetylated derivative chitosan, has numerous applications in various fields, e.g. in food, agriculture, cosmetic, biomedicine, textile, water treatment and pharmacy [1, 3-5]. In the processing of shrimp, a small part of the waste is dried and utilized [6], while the rest is dumped into the sea, which is one of the main pollutants in coastal areas [7, 8]. The utilization of shellfish waste has been proposed not only to solve environmental problems, but as a waste treatment alternative to the disposal of shellfish wastes [9].
Conventionally, isolation of chitin from marine waste material involves acid treatment to dissolve calcium carbonate (demineralization) followed by alkaline extraction to solubilize proteins (deproteinization) [3]. The conventional demineralization process of crustacean waste is costly and causes environmental problems. Hydrochloric acid is the most commonly used chemical in the demineralization of crustacean waste. The use of this strong acid: (1) harms the physiochemical properties of chitin, (2) results in a harmful effluent wastewater and (3) increases the cost of chitin purification process. Percot et al. [10] reported that using HCl for the demineralization of chitin results in detrimental effects on the molecular weight and the degree of acetylation that negatively affects the intrinsic properties of the purified chitin. They elaborated on the importance of the optimization of the extraction process parameters (pH, time, temperature and solids to acid ratio) in order to minimize chitin degradation and bring the impurity levels down to the satisfactory level for specific applications.
Mahmoud et al. [11] reported that the effectiveness of using lactic and/or acetic acids for demineralization of shrimp shells was comparable to that of using hydrochloric acid and other benefits may include (1) organic acids are less harmful to the environment (2) can preserve the characteristics of the purified chitin and can be produced from low cost biomass such as cheese whey (3) the resultant organic salts from the demineralization process can be used as a food preservative and/or an environmentally friendly de-icing/anti-icing agents.
Ameh et al [12] reported the kinetics of demineralization of shrimp shells using lactic acid and reported that the ash layer diffusion controlled shrinking core model was a good approximation for the demineralization process.
The aim of this research was to investigate the effect of variable citric acid concentrations on the kinetics of shrimp shell demineralisation.
Material and method
Shrimp was obtained from Kaduna central market and taken to the department of biological science, Ahmadu Bello University for identification. The exoskeleton of the shrimp was manually removed, washed dried and ground and sieved using a 250µm mesh. Citric acid 'AnalaR' (BDH Chemicals) was procured and prepared into five concentrations (0.1, 0.2, 0.3, 0.4 and 0.5M). The choice of acid concentration (0.1, 0.2, 0.3, 0.4 and 0.5M) was intended to give 50% deficit, stoichiometric, 50% excess, 100% excess and 150% excess respectively.
Demineralization
Various concentrations of citric acid solution (0.1M, 0.2M, 0.3M, 0.4M and 0.5M) were prepared by dissolving citric acid in distilled water. The prepared shrimp shells were then placed in a 1000ml conical flask and quickly mixed with 0.1M citric acid solution in the mass to volume ratio of 1:13. The content of the conical flask was maintained for 5 minutes, with stirring using a magnetic stirrer. After the given time interval, the content of the conical flask was quickly filtered using a muslin cloth. The solid residue was then washed severally with distilled water, until neutrality, as determined by the unchanged colour of blue litmus paper and a pH meter (Kent EIL 7055). The demineralized samples were dried and weighed. This was repeated in turns for other reaction times of 10, 15 and 20 minutes.
The entire procedure was then repeated for the other citric acid concentrations of 0.2, 0.3, 0.4 and 0.5M.
The concentration of calcium, in the raw as well as demineralised solid materials, was determined using AAS analysis (Atomic Absorption Spectrometer, Varian AA240FS).
Kinetic modelling
The shrinking core model (SCM) was considered as it models fluid-particle reactions [17]. The models for the various SCM control mechanism are:
1. For fluid-film diffusion control (FDC)
t/τ = XB (1)
2. For ash layer diffusion control (ALD)
t/τ = 1 – 3(1-XB)2/3 + 2(1 – XB) (2)
3. For chemical reaction control (CRC)
t/τ = 1 – (1 – XB)1/3 (3)
where τ is the time for complete reaction; XB is the conversion of Calcium
The right hand side of Equation 1, 2 and 3 where plotted against t and the fit R2 computed.
Results and discussions
Figure 1 presents the progression in the mass of shrimp shells with time for the various citric acid concentrations considered.
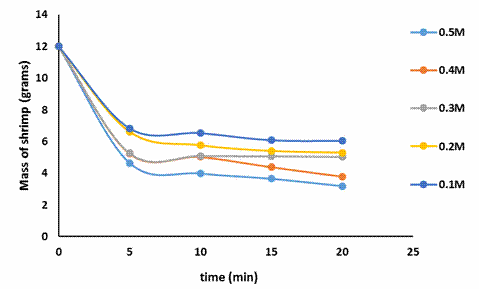
Figure 1. Effect of citric acid demineralization on the mass of shrimp shell
Figure 1 showed a progressive decrease in the concentration of calcium for all citric acid concentrations with time. Increasing acid concentrations resulted in an increase in the conversion or amount of calcium removed. For instance, after 20 minutes of demineralization the conversion of calcium for 0.1M and 0.5M citric acid solutions where 60% and 83% respectively. This was expected owing to the greater reactivity and availability of the acid.
Figure 2 presents the progression in the concentration of Calcium in the demineralised shrimp shells with time for the various citric acid concentrations considered.
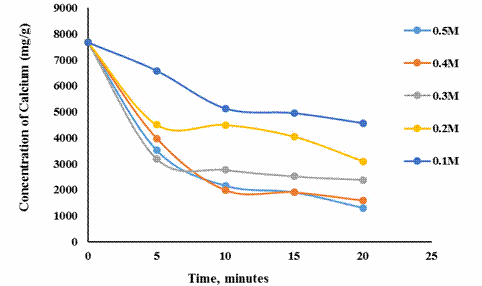
Figure 2. Effect of citric acid treatment on the Ca content of demineralised shrimp shell
Similar reason may be advance for Figure 2 which showed generally a progressive decrease in the mass of solid residue (after demineralization) with increasing acid concentration.
Figures 3 – 7 present the fitting of the kinetic data to the shrinking core model
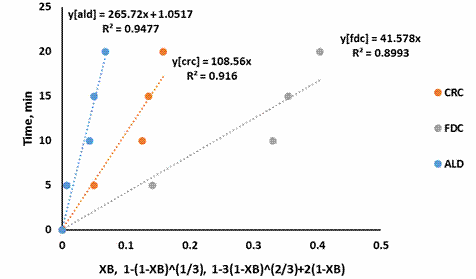
Figure 3. SCM for shrimp demineralization using 0.1M citric acid solution
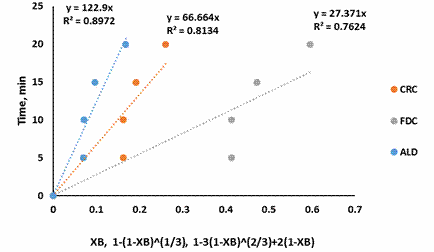
Figure 4. SCM for shrimp demineralization using 0.2M citric acid solution
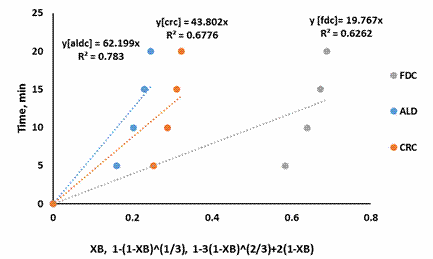
Figure 5.SCM for shrimp demineralization using 0.3M citric acid solution
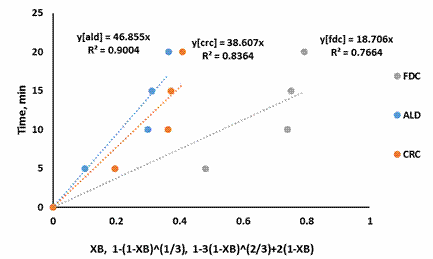
Figure 6. SCM for shrimp demineralization using 0.4M citric acid solution
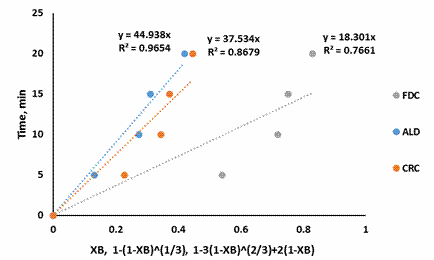
Figure 7. SCM for shrimp demineralization using 0.5M citric acid solution
Figure 3-7 showed that the Ash Layer Diffusion Control mechanism (ALD) gave a better approximation of the demineralization process compared to Fluid Film Diffusion control (FDC) or Chemical Reaction Control (CRC) mechanism. The relative magnitude of the R2 is indicative of this: for all citric acid concentrations considered, the R2 values were highest for ALD models. Treatment with 0.5M citric acid resulted in an R2 value of 0.9654 for ALD mechanism. For ALD reaction, it is visualised that a wall of ash (non-reactive material) prevent the fluid from moving freely to the zone of reaction (that is the unreacted core). In the demineralization of shrimp shells, other unreactive materials such as protein and chitin etc. are present, which can offer this resistance.
The results of this investigation are similar to the previous work done with lactic acid in the demineralization of shrimps by Ameh et al [12]. This was expected as the shrimp shell used in both investigations had not been deproteinized: which would contribute to ash diffusion resistance. Also the results of this investigation is widely different from that reported by Ameh et al [14] in which it was reported that the CRC mechanism was rate limiting. This again was expected as the investigation of Ameh et al [14] was based on the demineralization of deproteinized shrimp shell in which case the protein inhibition was no longer existing, having being removed by alkali treatment prior to demineralization.
The shrinking core model also supposes a large excess of the reacting fluid. Demineralization using 0.5M citric acid should be approaching this condition as 150% excess acid was used. The R2 value for this condition (0.9654), as well as the improvement in R2 as the acid concentration increased, from 0.2M to 0.5M, indicates that the shrinking core model is a good approximation for this system.
The reaction of calcium and acetic acid may be given as:
2C6H8O7 + 3Ca2+ → Ca3(C6H5O7)2 + 3CO2 + 3H2O (4)
In the demineralization of shrimp shells, the use of citric acid may have the following advantages (1) it has low toxicity Soccol et al [15] (2) citric acid can be produce from biomass (3) it will have less adverse effect on synthesized chitin and (4) the resultant organic salts Ca3(C6H5O7)2 (calcium citrate) from the demineralization process can be used as a food additive.
Conclusion
Demineralization of shrimp’s exoskeleton was carried out using 0.1M, 0.2M, 0.3M, 0.4M and 0.5M citric acid solution, which is expected to have some economic and environmental benefits. Analysis of the kinetic data using the shrinking core model indicated that the reaction was largely ash layer diffusion controlled as indicated by the magnitude of the R2 (0.965) when excess acid (150% excess) was used.
Acknowledgement
The authors wish to acknowledge the Multi-user Research Laboratory, Ahmadu Bello University for allowing the use of the Atomic Absorption Spectrometer.
References
1. Choorit W., Patthanamanee W., Manurakchinakorn S., Use of response surface method for the determination of demineralization efficiency in fermented shrimp shells. Bioresource technology, 2008, 14(99). p. 6168-6173.
2. Ravi Kumar M.N.V., 2000. A review of chitin and chitosan applications. Reactive and functional polymers, 1(46), p. 1-27.
3. Rinaudo, M., Chitin and chitosan: properties and applications. Progress polymer science, 2006, 7(31), p. 603-632.
4. Shirai K., Guerrero I., Huerta S., Saucedo G., Castillo A., Obdulia Gonzalez R. Hall G.M., Effect of initial glucose concentration and inoculation level of lactic acid bacteria in shrimp waste ensilation, Enzyme microbial technology, 2001, 4-5(28), p. 446-452.
5. Kim W.J., Lee W.G., Theodore K., Chang H.N., Optimization of culture conditions and continuous production of chitosan by the Fungi, Absidia Coerulea, Biotechnological bioprocessing engineering, 2001, 1(6), p. 6-10.
6. Xu Y., Gallert C., Winter J., Chitin purification from shrimp wastes by microbial deproteination and decalcification, Applied microbiological biotechnologies, 2008, 79, p. 687–697.
7. Zhai X., Hawkins S.J., Interactions of 1aquaculture and waste disposal in the coastal zone, Journal Ocean University, China, 2002, p. 8–12.
8. Gimeno M., Ramírez-Hernández J.Y., Mártinez-Ibarra C., Pacheco N., García-Arrazola R., Bárzana E., Shirai K., One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes, Journal of agricultural food chemistry, 2007, 55, p. 10345–10350.
9. Wang S.L., Chang T.J., Liang T.W., Conversion and degradation of shellfish wastes by Serratia sp. TKU016 fermentation for the production of enzymes and bioactive materials, Biodegradation, 2010, 21, 321–333.
10. Percot A., Viton C., Domard A., Optimization of chitin extraction from shrimp shells, Biomacromolecules, 2003, 4, p.12-18.
11. Mahmoud N.S., Ghaly A.E., Arab F., Unconventional approach for demineralization of deproteinized crustacean shells for chitin production, American journal of biochemistry and biotechnology, 2007, 3 (1), p. 1-9.
12. Ameh A.O., Abutu D., Isa M.T., Rabiu U., Kinetics of demineralization of shrimp exoskeleton using Lactic acid, Leonardo electronic journal of practices and technologies, 2014, 24, p. 13-22.
13. Levenspiel O., Chemical reaction engineering, 3rd Edition, John Wiley and Sons, New York, 1999, p. 566–576.
14. Ameh A.O., Isa M.T., Adeleye T.J., Adama K.K., Kinetics of demineralization of shrimp exoskeleton in chitin and chitosan synthesis, Journal of chemical engineering and material science, 2013, 4(3), p. 32-37.
15. Soccol C.R., Vandenberghe L.P.S., Rodrigues C., Pandey A., New perspectives for citric acid production application, Food technology and biotechnology, 2006, 44(2), p. 141 – 149.