The design of a low-cost device for the production of hydrogen
Amal NASSAR*, Eman NASSAR
Department of Mechanical Engineering, Higher Technological Institute, Tenth of Ramadan City, Egypt
E-mails: amal.nasser@hti.edu.eg; eman.nasser@hti.edu.eg
* Corresponding author, Phone: +201006414107; Fax: +20224924319
Abstract
The objective of this project is to design a generator for hydrogen gas. In this generator no need to storage the output gas, by controlling in the gas flow rate (according to usage needs) so, we did not need to hydrogen storage. The prototype is developed and tested and the idea can implement practically to save time and money.
Keywords
Generator design; Low-cost device; Hydrogen; Aluminium can
Introduction
Over the past years, it is becoming more likely that the emphasis on cleaner fuel will lead to the use of hydrogen in a significant way. Hydrogen is a chemical element with the chemical symbol H and atomic number 1. With an atomic weight of 1.00794, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the universe unlike oxygen, hydrogen is not found as free in the nature at any significant concentration, constituting roughly 75% of all baryonic mass. Hydrogen is the first element on the periodic table, making it the lightest element on earth. Since hydrogen gas is so light, it rises in the atmosphere and is therefore rarely found in its pure form, H2 [1].
Hydrogen fuel is a zero-emission fuel, which uses electrochemical cells, combustion in internal engines, to power vehicles and electric devices. It is also used in the propulsion of spacecraft and can potentially be mass-produced and commercialized for passenger vehicles and aircraft [2]. Hydrogen energy is environment-friendly. Because of the actual human ecological concerns, the exploitation of hydrogen as a universal fuel would be greatly acclaimed. During the last two decades or so, the elaboration of a hydrogen-based economy has made important progress on account of numerous research projects such as the hydrogen fuel cell and the hydrogen car [3].
Hydrogen is produced using both renewable and non-renewable resources, through various process routes. The available technologies for hydrogen production are reforming of natural gas; gasification of coal and biomass; and the splitting of water by water-electrolysis, photo-electrolysis, photo-biological production, water splitting thermochemical cycle and high temperature decomposition. The principal methods for the production of hydrogen involve water-electrolysis and natural gas reforming processes [4]. The worldwide increasing demand of hydrogen, such as in hydrogen fuel cells, has made it crucial to find hydrogen generation methods from inexpensive simple processes, many researchers suggested some innovative routes. Interestingly, a large number of the methods included sodium hydroxide as an essential ingredient. The use of sodium hydroxide for production of hydrogen is not new and was in an application even during 19th century [5]. Metallic aluminum has a high potential with a large chemical energy Therefore, the purpose of this study is to design a prototype for hydrogen gas generator. In this generator no need to storage the output gas, by controlling in the gas flow rate (according to usage needs) so, we did not need to hydrogen storage Figure 1 illustrates the schematic for the suggested generator.
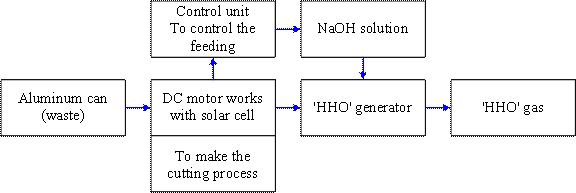
Figure 1. Schematic diagram for the hydrogen generator
Material and method
Design of the aluminium can chopped
The chopper will be used to convert aluminium can from the natural size of small pieces (≈ 20 mm). Chopper consists of 4 cutters (TCT blades) and shaft as shown in figure 2. The cutters got its motion from pulley connected with DC motor. TCT blade is a tungsten carbide-tipped TCT Cold saw blades are used to cut metal using a relatively slow rotational speed.

Figure 2. TCT cold saw blades
Pulley design
There are various factors influencing the pulley diameter. The pulley diameter is mainly determined by the conveyor belt class, but the required shaft diameter also influences the diameter. A golden rule for the pulley diameter is that it should be at least three times the diameter of the shaft [6]. The shaft diameter in our case is equal 12 mm so, the pulley diameter will be 39 mm.
Shaft design
There are three main factors that influence shaft design: Bending from the tensions on the conveyor belt, torsion from the drive unit and deflection. The shaft therefore needs to be designed considering all three of these elements. For the design of the shaft, based on bending and torsion, a max stress is used. This stress varies according to the material that is used for the shaft or according to the max stress allowed by the end user. We select BS 970 080M40 Carbon Steel as a material for the shaft. Table 1 and table 2 shows the Chemical composition and mechanical properties for the selected material. The safety factors for shaft design is (Kt 25 to 1.4) = 1. [5]. From the available data the shaft diameter equal 13 mm.
Table 1. Chemical composition of BS 970 080M40
|
From |
Value |
|
Carbon, C |
0.36 - 0.44 % |
|
Iron, Fe |
Balance |
|
Manganese, Mn |
0.60% |
|
Molybdenum, Mo |
<= 0.15 % |
|
Phosphorous, P |
<= 0.050 % |
|
Silicon, Si |
0.10 - % |
|
Sulfur, S |
<= 0.050 % |
Table 2. Mechanical properties of BS 970 080M40
|
Mechanical Properties |
Value |
|
Tensile Strength, Ultimate |
|
|
Tensile Strength, Yield |
|
|
Elongation at Break |
7.00% |
|
Hardness, Brinell |
146 |
Design of the sodium hydroxide mixer
Sodium hydroxide (caustic soda) will mix with water to reduce its concentration and converting its liquid state. Liquid caustic soda is a corrosive product Mixing process make in containers made from Mild steel. Figure 3 shows the Container, we used a butt-welding to weld the container edges. The mixing operation generates some heat, but it is not exceeded 49°C, so no need to make annealing to prevent stress corrosion [8]. The mixer was made from Mild steel (4 blade stirrer welded together) as shown in figure 4. Table 3 and Table 4 shows the Chemical composition and mechanical properties for the selected material [7].
Table 3. Chemical composition of the Mild steel
|
From |
Value |
|
Carbon |
0.17% |
|
Silicon |
0.33% |
|
Manganese |
0.75 % |
|
Sulfur |
0.035% |
|
Phosphorus |
0.029% |
Table 4. Mechanical properties of Mild steel
|
Value |
|
|
Max Stress |
540 n/mm2 |
|
Yield Stress |
430 n/mm2 Min |
|
0.2% Proof Stress |
410 n/mm2 Min |
|
Elongation |
10 % Min |
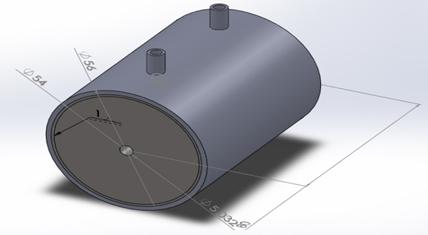
Figure 3. The container
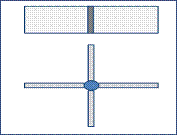
Figure 4. Four- blade stirrer used to mix sodium hydroxide with water.
Selection of the DC Motor
We need a reliable, time-tested and low cost motor, and then brushed DC motor technology may be what we looking for. Initial Motor Choice Parameters: Available voltage (V) =20V; Output torque required (M) 6N-M; Output speed required (n) =2000 RPM.
As a first step, determine the required output power as follows:
Po = n.M/1350
P=1. 25 k. Watts
The motor should be rated at least 1.5 to 2 times the desired output power in relation to its maximum output power (at nominal voltage). A motor with approximately 1.9 to 12.5 k. Watts maximum output power should suffice. The electric motor is expected to provide the torque required to rotate 2 pulleys (to rot the mixer and cutter).
Description of the machine
The machine consists of an electric motor to give the rotation motion of the mixer and of the cutter. Water and sodium hydroxide feed will be controlled by using micro controller that controls in gates motion. Micro controllers will also control the reaction process between aluminium is and sodium hydroxide. Figure 5 shows the assembly of the hydrogen generator, pieces and sodium hydroxide controlling by the motion of the gates. When the electronic gates open for a certain period, the feeding process happens to aluminium piece.
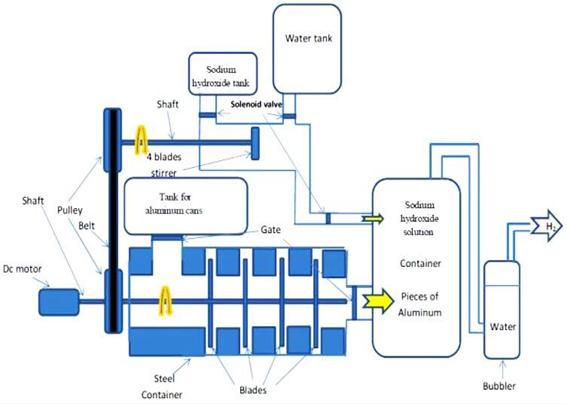
Figure 5. The main components of the hydrogen generator
Result and discussion
Measuring the gas flow rate
To measure the gas flow rate, we did a simple experiment to measure the flow rate of gas output at (0.25 gm from aluminum for each 5 cm3 from sodium hydroxide solution). Figure 6 illustrates the flow rate test. The experimental result of volume flow rate was 9.2 L/min.
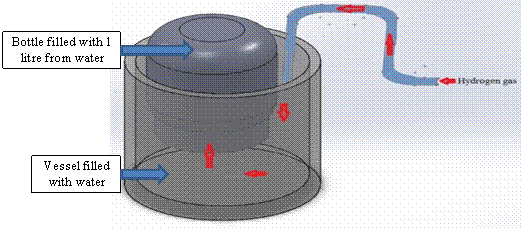
Figure 6. Flow rate test
Volume flow rate calculation
In this section we calculate the mass flow rate for hydrogen gas from equation 1.
|
M = ρ · v · A v = 8 m/s A = D2π A = 0.0082 · π =2 · 10-4 m2 ρ = 0.535 kg/m3 |
(1) |
The mass flow rate = 8.56 · 10-5
Volume flow rate = m/ρ = (8.56 ·10-5)/0.535 = 1.6 · 10-4 m3/s = 9.6 L/min
Conclusion
An automatic system to produce hydrogen gas has been designed and developed. The system consists from system to control in sodium hydroxide and aluminum pieces reaction. By controlling in this reaction the gas flow rate can change. A micro controller will be utilized to control the whole process. In the suggested generator no need to storage the output gas, by controlling in the gas flow rate (according to usage needs) so, we did not need to hydrogen storage.
References
1. Palmer, D, Hydrogen in the Universe NASA. 2008, pp 30
2. Altork L.N., Busby J. R., Hydrogen fuel cells: part of the solution, International technology and engineering educators association, 2010, 70 (2), p. 22-27.
3. Andersen E.R., Frederikstad (NO); Andersen E.J., New Denmark (CA), Method for producing hydrogen, Patent application publication, United States, 2003. PN. US6638493 B2.
4. Probstein R.F., Hicks R.E., Synthetic fuels, 3rd Ed. New York, Dover publications; 2006, p. 510.
5. Kumar S., Saxena S. K., Role of sodium hydroxide for hydrogen gas production and storage, Materials and processes for energy: communicating current research and technological developments FORMATEX 2013
6. Available at: http://www.bosworth.co.za/downloads/conveyor-pulley-design.pdf
7. Available at: http://www.matweb.com/search/datasheettext.aspx?matid=10335
8. Operating efficiently operating safely caustic soda solution handbook. The dow chemical company. 2013