Electrochemical determination of phenol at natural phosphate modified carbon paste electrode
Tarik EL OUAFY, Abdelilah CHTAINI, Hassan OULFAJRITE, Rachida NAJIH
Equipe dElectrochimie Moléculaire et Matériaux Inorganiques, Faculté des Sciences et Techniques de Beni Mellal, Université Sultan Moulay Slimane
E-mail: a.chtaini@usms.ma
*Corresponding author: Pr. A. Chtaini, BP: 523, Beni Mellal, Maroc
Abstract
A Cyclic voltammetry (VC) and Square Wave Voltammetry methods for the determination of trace amounts of phenol at carbon paste electrode modified with Natural Phosphate (NP-CPE) is proposed. The results showed that the NP-CPE exhibited excellent electro catalytic activity to phenol. The concentration of phenol and measuring solution pH was investigated. This electrochemical sensor shows an excellent performance for detecting phenol.
The sensor was successfully applied to the determination of phenol in a real sample with satisfactory results.
Keywords
Modified electrodes; Cyclic voltammetry; Natural phosphate; Phenol
Introduction
Phenol and related compounds are used extensively in industry in the manufacture of a large variety of aromatic compounds including rubber, fertilizer, paints, drug preparations, petroleum, and agricultural industries [1-3]. Phenol is reported to be carcinogenic and exposure to phenol results in several symptoms such as convulsions, dizziness and irregular respiration [1, 4].
The phenols are noted more as water pollutants than as air pollutants [5]. It is frequently pollutant in industrial waste and occurs in soil and drinking water supplies [6]. In the food industry, phenols are of interest because they are essential compounds of fruit juices, beer, and wines [7]. Since many phenolic compounds can cause bad taste and undesirable odour contamination and are highly toxic and hazardous to human health [8], their analysis at low concentrations is very important [9, 10]. As the manufacture and use of phenols requires qualitative and quantitative control, a wide variety of methods have been developed to determine phenolic compounds. The range of available methods extends from distillation [11], membrane extraction [12], liquid-liquid extraction [13, 14] and gas chromatography [15, 16] to more sophisticated techniques such as microwave-assisted extraction [17, 18], ultra-sonication and supercritical fluid extraction [19, 20].
Recently, some achievements in the field of enzyme electrode [21, 22] and biosensors [23, 24] have been reported. Tyrosinase-based biosensors for the determination of phenolic compounds in the organic phase have been reported extensively [25-27]. Regarding electro analytical techniques, procedures involving phenol oxidation at solid electrodes [28-29] have been reported. In addition, chemically-modified carbon paste electrodes have proven very useful for analytical applications [22, 30]. In the previous works [31-35], the electrochemical oxidation of phenol was investigated we present a simple and sensitive method of determination of these compounds based on their reaction. The aim of the work reported here was to investigate the electrochemical properties of phenol on Natural phosphate modified carbon paste electrode as well as the electrochemical characterization of electrodes by cyclic voltammetric technique.
Material and method
Instrument
Voltammetric experiments were performed using a voltalab potentiostat (model PGSTAT 100, Eco Chemie B.V., Utrecht, The Netherlands) driven by the general purpose electrochemical systems data processing software (voltalab master 4 software) run under windows 2007. The three electrode system consisted of a chemically modified carbon paste electrode as the working electrode a saturated calomel electrode (SCE) serving as reference electrode, and platinum as an auxiliary electrode.
Electrodes
Modified electrodes were prepared by mixing a carbon powder and the desired weight of Natural Phosphate (NP). The body of the working electrode for voltammetric experiments was a PTFE cylinder that was tightly packed with carbon paste. The geometric area of this electrode was 0.1256 cm2. Electrical contact was made at the back by means of a bare carbon.
Procedure
The initial working procedure consisted of measuring the electrochemical response at
NP-CPE at a fixed concentration of phenol. Standard solution of phenol was added into the electrochemical cell containing 100 mL of supporting electrolyte.
The mixture solution was kept for 20 s at open circuit and deoxygenated by bubbling pure nitrogen gas prior to each electrochemical measurement.
The cyclic voltammetry was recorded in the range from -1.8V to 1.8V.
Optimum conditions were established by measuring the peak currents in dependence on all parameters. The square wave voltammetry was recorded in the range from -1.8V to1.8V, for which the scan rate is 1 mV.s-1, step potential 50 mv, amplitude 2 mV and duration 0.1 s. Optimum conditions were established by measuring the peak currents in dependence on all parameters. All experiments were carried out under ambient temperature. All experiments were carried out under ambient temperature.
Results and discussion
Surface characteristics
The surface structure of natural phosphate (NP) electrode was observed using scanning electron microscopy (Figure 1).
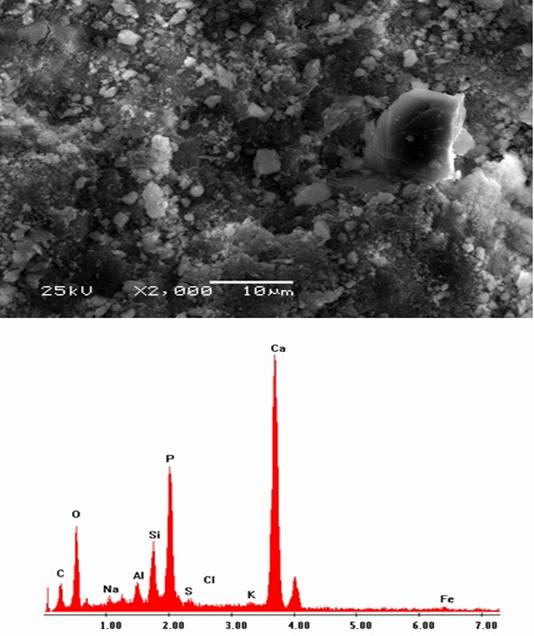
Figure 1. Scanning electron micrograph of natural phosphate
Phenol oxidation
Cyclic voltammetry at a scan rate of 100mV/s was the electrochemical technique applied to study the oxidation behaviour of phenol. Figure 2 shows the oxidation peak of the phosphate modified electrode paste appeared at approximately 1 V in a phenol concentration of 0.004 M. No reduction peak was observed.
Figure 3 shows the phenol undergone oxidation with the loss of one electron and hydrogen transfer. The presence of only anodic oxidation peak year, suggests that the electrochemical process of phenol is totally irreversible.
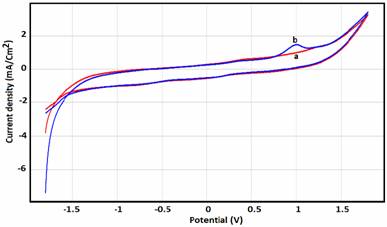
Figure 2. CVs recorded for 4 mM phenol at pH=5 at bare NP-CPE (a) and NP-CPE/phenol
(b), scan rate 100 mV/s, preconcentration time (tp)= 4min
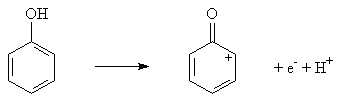
Figure 3. The electrochemical oxidation reaction of phenol
Optimization of experimental conditions
Optimum conditions for the electrochemical response were established by measuring the peak current in dependence on all parameters.
Influence of accumulation time
The effect of the accumulation time is investigated (Figure 4); this significantly affects the oxidation peak current of phenol.
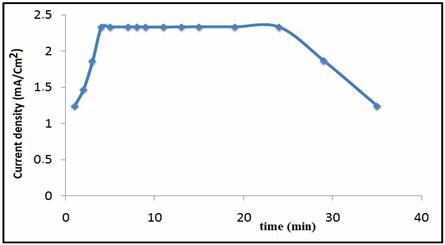
Figure 4. Effects of accumulation time on oxidation peak currents of 3 mmol L−1
phenol (pH=5) at NP-CPE, supporting electrolyte is Na2SO4 0.1M
The peak current of 3 mmol L−1 phenol increases greatly within the first 3min. Further increase in accumulation time does not increase the amount of phenol at the electrode surface owing to surface saturation, and the peak current remains constant. This phenomenon is due to the cavity structure of phosphate-CPE that improves the ability of the electrode to adsorb electro active phenol. Maybe this is attributed to the saturated adsorption of phenol on the NP-CPE surface. Taking account of sensitivity and efficiency, accumulation time was 4 min in the following experiments.
Effect of scan rate
The influences of scan rate on the oxidation peak potential (Ep) and, peak current (Ip) of phenol, (0.1M Na2SO4, pH=5) were studied by cyclic voltammetry. The figure 5 shows both the anodic currents linearly increase with the scan rate over the range of 40 to 140mVs-1, suggesting that the electron transfers for phenol at the phosphate modified CPE is adsorption controlled reaction.

Figure 5. CVs acquired on NP-CPE with 8 mM phenol in the buffer solution at different scan rates from 40 to 140mV.s-1. Inset is the plot of the peak current of phenol versus scan rate
The figure 6 shows the linear relationship between the scan rate anodic peak currents of phenol at NP/CPE.
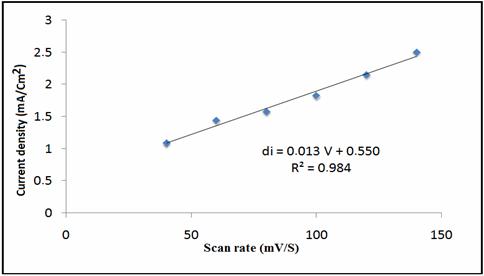
Figure 6. Plot of peaks area versus scan rate
Calibration graph
In order to obtain an analytical curve for the developed sensor, we carried out cyclic voltammograms for oxidation a phenol at different concentrations in 0.1mol L−1 Na2SO4 (pH=5) at a sweep rate of 100 mVs-1.
Figure 7 shows the CV curves of different concentration of phenol at NP/CPE was increased from 2 mM to 12 mM. The anodic peak current increases linearly with the concentration of phenol and the plot of current versus concentration obeys Randles-Sevic equation, which implies that the electrode process is adsorption controlled reaction.
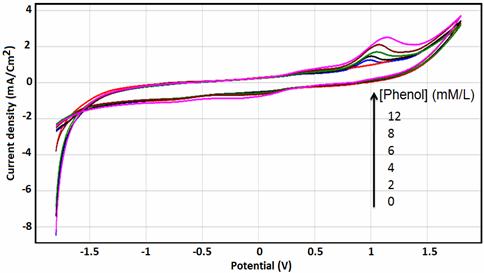
Figure 7. Cyclic Voltammograms of different concentration of phenol (2mM to
12mM) at NP/CPE in 0.1 M Na2SO4 PH=5, Scan rate 100 mV/s
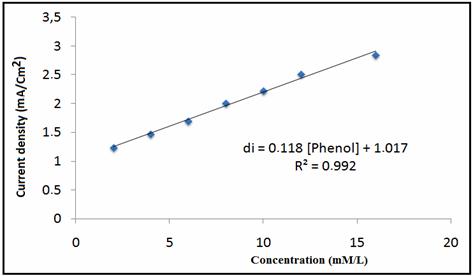
Figure 8. Plot of peaks area versus added concentration of phenol
Influences of pH
The effect of varying pH on the current response of NP/CPE at constant phenol concentration (16 mM) is shown in Figures 9 and 10. As can be seen, the peak current gradually reduces with the increase of pH and reaches a minimum value when the pH is 7.0. Further increase in the solution pH yields a gradual increase in the phenol peak current.
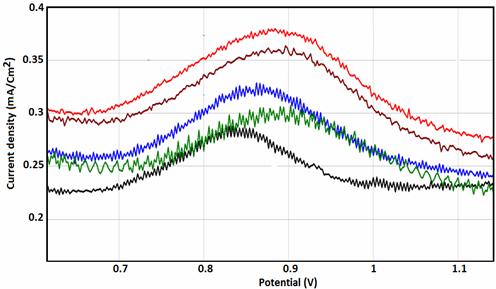
Figure 9. Effect of pH on the oxidation of phenol at the phosphate modified CPE
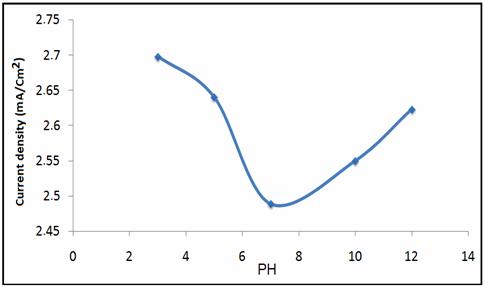
Figure 10. Plot of the relationship between solution pH and the oxidation peak Current
Practical application
In order to evaluate the performance of NP modified carbon paste electrode by practical analytical applications, the determination of phenol was carried out in tap water without any pre-treatment. No phenol traces were found when the proposed procedure was used. The analytical curves were obtained by CV experiments in supporting electrode (Figure 10). It was founded that the peaks currents increase linearly versus phenol added into the tap water (Figure 11).

Figure 10. Cyclic Voltammograms of different concentration of phenol (2mM to
10mM) at NP/CPE in 100ml tap water, Scan rate 100 mV/s
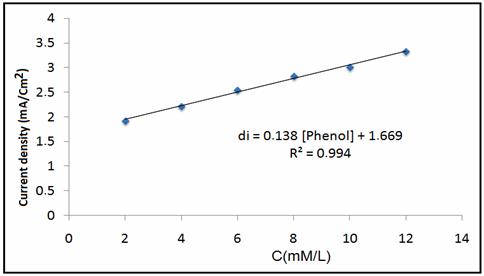
Figure 11. Plot of peaks area versus added concentration of phenol
Conclusions
Cyclic voltammogram of phenol detection using phosphate modified carbon paste electrode showed an irreversible response. Phenol in solution was only oxidized and not reduced.
The fact that the modified electrode worked best at pH=3.
Since only the modified electrode was able to detect the presence of phenol, this lead to a conclusion that phosphate modified carbon paste provided a suitable surface for electron transfer. Due to contamination of electrolyzed component on the electrode surface, it is highly discouraged to reuse the electrode. Emphasis on environmental applications of this sensor can be expounded upon as an in-class exercise.
References
1. Patty F.A., Industrial hygiene and toxicology, Interscience, 1963, 2, p.1363.
2. Kolthoff I.M.; Elving P.J.; Stross F.H., Treatise on analytical chemistry, Wiley, 1971, 2, p.490.
3. Leithe W., Analysis of air pollutants,
4. Leithe W., Analysis of organic pollutants in water and waste water, Ann Arbor science, Ann Arbor, MI, 1972, p.113.
5. Manahan S.E., Fundamental of
environmental chemistry, Lewis,
6. Morales A.; Birkholz D.A.; Hrudey S.E., Analysis of pulp mill effluent contaminations in water, sediment and fish bile fatty and resin acids, Water environ. res., 1992, 64, p.660.
7. Buckee G.K., Determination of the volatile components of beer, J. inst. brew, 1992, 98, p.78.
8. Chen D.; Ray A.K., Photodegradation kinetics of 4-nitrophenol in TiO2 suspension, Water res, 1998, 32, 3223. Sensors 2004, p.4179
9. Realini P.A., Determination of priority pollutant phenols in water by HPLC, J. Chrom. Sci, 1981, 19, p.124.
10. Tyagi R., Determination of substituted phenols in water by gas chromatography/mass spectroscopy after solid phase extraction, Fresenius environ. bull., 1995, 4, p.751.
11. Green J.P.; Strierwalt B.K.; Green J.A.; Grizzle P.L., Analysis of polar compound classes in SRC II liquids-comparison of non-aqueous titrametric, I.R. spectrometric and H.P.L.C. methods, Fuel, 1985, 64, p.1571.
12. Gonzalo E.R.; Perez-Pavon J.L.; Ruzicka J.; Christinan G.D., Flow injection analysis determination of phenols in kerosene and naphtha by membrane extraction preconcentration, Anal. chim. acta, 1992, 259, p.37.
13. Heemken O.P.; Theobald N.; Wenclawiak B.W., Comparison of ASE and SFE with soxhlet, sonication and mathematic saponification extraction for the determination of organic micropollutants in marine particulate matter, Anal. chem., 1997, 69, p.2171.
14. Snyder J.L.; Grob R.L.; McNally M.E.; Oostdyk T.S., Comparison of supercritical fluid extraction with classical sonication and soxhlet extractions for selected pesticides, Anal. chem., 1992, 64, p.1940.
15. Lompart M.P.; Lorenzo R.A.; Cela R., Multivariate optimization of supercritical fluid derivatization and extraction of phenol in soil samples, J. chrom. sci., 1996, 34, p.43.
16. Lompart M.P.; Lorenzo R.A.; Cela R., Optimization of supercritical fluid extraction of Phenol and cresols in soil samples, J. chrom, A, 1996, 723, p.123.
17. Lompart M.P.; Lorenzo R.A.; Cela R.; Li K.; Belanger J.M.R.; Pare J.R.J., Evaluation of supercritical fluid extraction, microwave-assisted extraction and sonication in the determination of some phenolic compounds from various soil matrics maria, J. chrom. a, 1997, 774, p.243.
18. Egizabal A.; Zuloaga O.; Etxebarria N.; Fernandez L.A.; Madariaga J.M., Comparison of microwave-assisted extraction and soxhlet extraction for phenols in soil samples using experimental designs, Analyst, 1998, 123, p.1679.
19. Asharf-Khorassani M.; Gidanian S.; Yamini Y., Effect of pressure, temperature, modifier, modifier concentration, and sample matrix on the supercritical fluid extraction efficiency of different phenolic compounds, J. chrom. sci., 1995, 33, p.658.
20. Santos F.J.; Jauregui O.; Pinto F.J.; Galceran M.T., Experimental design approach for the optimization of supercritical fluid extraction of chlorophenols from pollutant soils, J. chrom. A, 1998, 823, p.249.
21. Deng Q.; Gue Y.; Dong S., Cyro-hydrogel for the construction of a tyrosinase-based biosensor, Anal. chim. acta, 1996, 319, p.71.
22. Gorton L., Carbon paste electrodes modified with enzymes, tissues, and cells, Electroanal, 1995, 7, p.23.
23. Sampath S.; Lev O., Membrane-free, rhodium-modified, methyl silicate-graphite Amperometric biosensor, J. electroanal. chem., 1997, 426, p.141.
24. Wang R.; Narang U.; Prasad P.N.; Bright F.V., Affinity of antifluorescein antibodies encapsulated within a transparent sol-gel glass, Anal. chem., 1993, 65, p.2671.
25. Dong S.; Guo Y., Organic phase enzyme electrode operated in water-free solvents, Anal. chem., 1994, 66, p.3895.
26. Schubert F.; Saini S.; Turner A.P.F.; Scheller F., Organic phase enzyme electrodes for the determination of hydrogen peroxide and phenol, Sens. actuators B., 1992, 7, p.408.
27. Conner M.P.; Sanchez J.; Wang J.; Smyth M.R.; Mannino S., Silicone-grease-based immobilisation method for the preparation of enzyme electrodes, Analyst, 1989, 114, p.1427.
28. Hedenburg J.F.; Freiser H., Anodic voltammetry of phenols. Anal. chem., 1953, 25, p.1355.
29. Smyth M.R.; Smyth W.F. Voltammetric methods for the determination of foreign organiccompounds of biological significance. A review, Analyst, 1978, 103, p.529.
30. Kalcher K., Chemically modified carbon paste electrodes in voltammetric analysis. Electroanal, 1990, 2, p.419.
31. Nematollahi D.; Hesari M., Electrochemical study of iodide in the presence of barbituric acid. Application to the catalytic determination of barbituric acid, J. anal. chem., 2001, 56, p.1109.
32. Nematollahi D.; Hesari M., Electrochemical study of iodide in the presence of barbituric acid.Application to coulometric titration of barbituric acid, Microchemical J, 2001, 70, 7.
33. M. El Mhammedi, M. Achak, A. Chtaini., J., of Hazardouz Materials, 2009, 161, p.55-61.
34. H. Massaï, B.B. Loura, M.J. Ketcha, A. Chtaini, Portugaliae electrochimica acta, 2009, 27(6), p.691-698.
35. Harouna Massai, Benguellah Benoitc, Mbadcam Joseph Ketcha and Abdelilah Chtaini Bulletin of
the catalysis society of