Review on phase change materials for building applications
Lavinia SOCACIU1*, Angela PLEŞA2, Paula UNGUREŞAN3, and Oana GIURGIU4
1,2,3,4 Department of Mechanical Engineering, Technical University of Cluj-Napoca, Romania
E-mails: 1Lavinia.socaciu@termo.utcluj.ro; 2Angela.plesa@termo.utcluj.ro; 3Paula.unguresan@termo.utcluj.ro; 4Oana.giurgiu@termo.utcluj.ro.
* Corresponding author, Phone: +40264401605
Abstract
In nowadays, the Phase Change Material (PCM) is a viable alternative for reducing the energy consumption and for increase the thermal comfort in buildings. The use of PCM in building applications provides the potential to increase the indoor thermal comfort for occupants due to the reduced indoor temperature fluctuations and lower global energy consumption. The possibility to incorporate the PCM into the material of construction for cooling and heating the buildings gained the interest of researchers from all the world because the PCM have a high heat of fusion, meaning it is capable to storing and release large amounts of energy in the form of heat during its melting and solidifying process at a specific temperature.
Keywords
Phase change material (PCM); Building applications; Thermal comfort; Thermal energy storage
Introduction
Phase Change Materials (PCMs) have been considered for thermal storage in buildings since before 1980. With the advent of PCM implemented in gypsum board, plaster, concrete or other wall covering materials, thermal storage can be part of the building structure even for light weight buildings [1].
PCMs are substances that release and absorb large amounts of heat during the phase change process, for example, from liquid to solid or from gas to liquid. For PCM applications in buildings, which operate in relatively low temperatures, around the ambient air temperatures, only solid-liquid and solid-solid phase change materials are of interest. Liquid-gas phase change materials would be impractical because of the large volume changes that would be required of the PCMs during the phase change processes [2].
Incorporating suitable phase change materials into the walls, ceiling and floor of buildings can render it feasible to capture solar energy directly and store significant amounts of thermal energy in the building envelope without the large structural mass associated with sensible heat storage, which helps to decrease the frequency of internal air temperature swings and maintain the temperature closer to the desired temperature for a longer period of time [3].
The aim of this research was to make a review of the phase change materials that can be incorporated in buildings material. This materials are often used in construction because of theirs properties of reducing the energy consumption and ensuring the thermal comfort.
Material and method
For beginning bibliographical references was search in Google Scholar database. The first interrogation was made using all the words: “phase change materials for building applications”. The inclusion criterion for the search was: anywhere in the article my words occur, and the exclusion criteria were: patents and citations. From first interrogation result a number of 3050000 bibliographical references.
The second interrogation was made using the exact phrase: “phase change materials for building applications” for reducing the number of results. The criterion for including and excluding bibliographical references was kept unchanged. From the second interrogation we obtained 148 results. Eight bibliographical references contain all words in the title of article was find. Three results were returned from the interrogation with the exact phrase in the title of article.
The next step was to search in database for the expression: “PCM for building applications”. The criterion for including and excluding bibliographical references was kept unchanged. The results obtained were: 47800 bibliographical references which contain in the text of article this expression and 3 articles which contain in the title of article this expression. The interrogation using the exact phrase: “PCM for building applications” generate 34 results which contain in title this expression and a number of one bibliographical references which contain the exact phrase in the title.
In the next step I studied the abstracts as well as the contents for bibliographic references available, and then for each reference I decided if it is significant or not for the field of research.
Results and discussion
PCMs for building applications
PCMs are generally divided into three main categories, organic PCMs, inorganic PCMs and eutectics of organic and inorganic compounds [4] as shown in figure 1 [5]. Table 1 and 2 present the advantages and disadvantages of organic and inorganic materials.
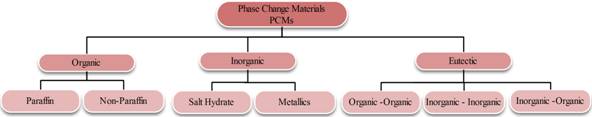
Figure 1. Types of PCM
Table 1. Advantages and disadvantages for organic materials
|
Advantages |
Disadvantages |
|
High specific heat [5]; Low or none under cooling [6]; Wide and variable melting point range [7]; Available in large temperature range (from 20°C up to 70°C) [5]; Thermally reliable in long run (freeze melting cycle) [5]; Low vapor pressure in melt form [5]; Reasonable latent heat of fusion (120kJ/kg up to 210 kJ/kg) [5]; Small volume change during phase transition [5]; Relatively high heat of fusion [7]; Chemical and thermal stability [6]; Non-corrosives [6]; Innocuous (neither toxic or irritant) [5]; Recyclable [5,7]; Compatible with construction materials [5]; Good compatibility with other materials [7]. |
Low thermal conductivity [6]; Lower phase change enthalpy [6]; Relative large volume change [7]; Non compatible with plastic containers [5]; Flammability [6]. |
Table 2. Advantages and disadvantages for inorganic materials
|
Advantages |
Disadvantages |
|
High latent heat of fusion [5,7]; High thermal conductivity [5,7]; Greater phase change enthalpy [6]; Sharp phase change [5]; Low volume change [7]; Non-flammable [5]; Low cost [7]; Compatible with plastic container [5]; Low environmental impact [5]. |
Under cooling [6]; High volume change [5]; Phase segregation, lack of thermal stability [6]; Poor nucleation rates [7]; High vapour pressure (induce water loss and cause progressive change in thermal behaviour during thermal cycling process) [5]; May show long term degradation by oxidation, hydrolysis, thermal decomposition and other reactions [5]; Exhibit variable chemical stability [5]; Corrosion [6] and irritant [5]. |
The most important properties of PCM are the temperature and the heat of fusion. For building applications the employed PCM should have a phase transition close to human comfort temperature (22-26ºC). In general, it is imperative to have the temperature of fusion within the temperature range of application, though the temperature of fusion as such does not affect the energy storage capacity of a material. However, as a phase change is involved in melting, the inclusion of the temperature of fusion in the temperature range of application can permit the use of phase change as an on-off switch [8].
The heat of fusion, also known as enthalpy of fusion or latent heat of fusion, refers to the amount of thermal energy that a material must absorb or evolve in order to change its phase from solid to liquid or vice versa. Storage capacity of the PCM is directly dependent on the heat of fusion, as large values of heat of fusion lead to more efficient systems [8]. Passive heating/cooling applications for buildings require high heat of fusion to increase the energy stored, reducing the peaks of heating/cooling demand [6]. In figure 2 and 3 is illustrated general behavior of organic and inorganic phase change materials in terms of melting point and range, specific heat and enthalpy.
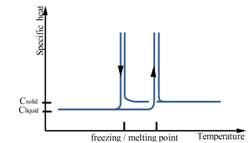
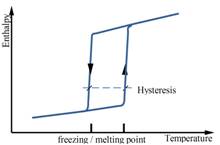
Figure 2. General behaviour of organic PCMs in terms of melting point and range, specific heat and enthalpy [9]
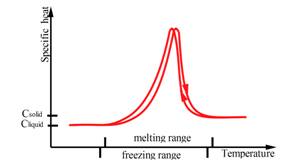
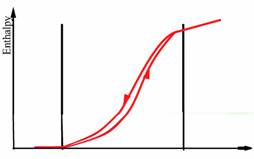
Figure 3. General behaviour of inorganic PCMs in terms of melting point and range, specific heat and enthalpy [9]
In order to be used for building applications, the PCM must possess certain desirable thermo physical, kinetic, chemical, economic and environmental properties (Table 3).
Table 3. Main criteria that governing the selection of PCMs
|
Thermo physical |
|
Phase change temperature suitable for building application, in the desired operating temperature range [5,9] |
|
High latent heat of fusion per unit volume so that smaller size of container can be used [5] |
|
High thermal conductivity to assist in charging and discharging of PCM within the limited time frame [5] |
|
High specific heat so that additional energy in the form of sensible heat is available to the thermal energy storage system [5] |
|
Small volume change during phase transition and small vapour pressure at operating temperature so as to avoid the containment problem [5] |
|
PCM should melt completely (i.e. congruent melting) during phase transition so that the solid and liquid phases are homogenous [5] |
|
Thermally reliable (i.e. cycling stability) so that PCM is stable in terms of phase change temperature and latent heat of fusion and can be used in long run [5] |
|
Good heat transfer [9] |
|
Small vapor pressure at operating temperature [9] |
|
High density [9] |
|
Favorable phase equilibrium and no segregation [9] |
|
Complete melting [7] |
|
Chemical properties |
|
Chemically compatible with construction/encapsulated materials [5] |
|
No degradation after large number of thermal (freeze/melt) cycles so as to assure long operation life [5] |
|
Non-toxic, non-flammable and non-explosive so as to assure safety [5] |
|
Corrosion resistant to construction/encapsulated materials [5] |
|
Complete reversible melt/freeze cycles [9] |
|
Chemical stability [7] |
|
High freeze/melt stability [7] |
|
Kinetic properties |
|
High rate of nucleation so as to avoid super cooling of the PCM in liquid phase [5,9] |
|
High rate of crystal growth so that heat recovery from the storage system is optimum [5,9] |
|
Economic properties |
|
Low cost [7] |
|
Abundant and commercially available [5,9] |
|
Environmental properties |
|
Low environmental impact and non-polluting during service life [5] |
|
Having recycling potential [5] |
|
Separation facility from the other materials [9] |
|
Low embodied energy [9] |
It is worth mentioning here that no PCM can have all the desirable properties. Therefore, the choice of a PCM for a given thermal energy storage application in buildings require careful examination of the thermo-physical, kinetic, chemical, economic and environmental properties of the various available candidates, comparing their merits and demerits and in some cases achieving a certain degree of compromise [5].
For including PCMs in building constructions, some specific thermal, physical, kinetic and chemical properties are desired:
Ø From a thermal point of view, a suitable phase change temperature range, a high latent heat of fusion and a good heat transfer towards the PCM are desired. The desired phase change temperature will depend on climatic conditions and the desired comfort temperature.
Ø From a physical point of view, favorable phase equilibrium, i.e. no phase segregation, a high density and small volume changes at the phase change are desired for easy incorporation in existing building materials or structures.
Ø From a kinetic point of view, no super-cooling and a sufficient crystallization rate are desired to make optimal use of the properties and possibilities of PCMs. Super-cooling, i.e. the process of lowering the temperature of a liquid below its freezing point without becoming a solid, could strongly affect the performance of the PCMs based on the chosen suitable phase change temperature by influencing this temperature.
Ø From a chemical point of view, a long-term chemical stability of the PCM despite cycling, compatibility with construction materials, non-toxicity and no fire hazard is desired [10].
PCMs are available commercially from a range of suppliers like: RUBITHERM® Technologies GmbH, EPS Ltd, Climator, Teap, Cristopia, Mitsubishi Chemical, Doerken and Merck. Table 4 presents the thermal properties of commercially available PCMs, with a melting point between 21 and 28°C. Rubitherm® Technologies GmbH specializes in paraffin-based PCM production for application as a thermal storage medium. The melting points of their products range from -10 to 100°C. EPS Ltd specializes in PCMs, environmental chemicals and services. They have a wide variety of containers and applications to house the PCM or can fill any given container with the specified PCM. PCM’s can operate in the temperature range from -114 to 164°C [7].
Table 4. Commercially available PCMs with melting point between 21 and 28°C
|
PCM |
Source |
Type |
Phase change temperature [°C] |
Latent heat capacity [kJ/kg] |
Density for solid [kg/m3] |
Specific heat capacity [kJ/kgK] |
Thermal conductivity [W/mK] |
Refs |
|
RT 21 HC |
Rubitherm |
Paraffin |
21 |
190 |
880 |
2 |
0.2 |
[11] |
|
RT 22 HC |
Rubitherm |
Paraffin |
22 |
200 |
760 |
2 |
0.2 |
[11] |
|
RT 24 |
Rubitherm |
Paraffin |
24 |
150 |
880 |
2 |
0.2 |
[11] |
|
RT 25 HC |
Rubitherm |
Paraffin |
25 |
230 |
880 |
2 |
0.2 |
[11] |
|
RT 27 |
Rubitherm |
Paraffin |
27 |
179 |
880 |
2 |
0.2 |
[11] |
|
RT 28HC |
Rubitherm |
Paraffin |
28 |
245 |
880 |
2 |
0.2 |
[11] |
|
PlusIce PCM S21 |
EPS Ltd. |
Hydrated Salt |
22 |
170 |
1530 |
2.2 |
0.54 |
[12] |
|
PlusIce PCM S23 |
EPS Ltd. |
Hydrated Salt |
23 |
175 |
1530 |
2.2 |
0.54 |
[12] |
|
PlusIce PCM S25 |
EPS Ltd. |
Hydrated Salt |
25 |
180 |
1530 |
2.2 |
0.54 |
[12] |
|
PlusIce PCM S27 |
EPS Ltd. |
Hydrated Salt |
27 |
183 |
1530 |
2.2 |
0.54 |
[12] |
|
PlusIce PCM A22 |
EPS Ltd. |
Organic solutions |
22 |
145 |
785 |
2.22 |
0.18 |
[12] |
|
PlusIce PCM A23 |
EPS Ltd. |
Organic solutions |
23 |
145 |
785 |
2.22 |
0.18 |
[12] |
|
PlusIce PCM A25H |
EPS Ltd. |
Organic solutions |
25 |
226 |
785 |
2.15 |
0.18 |
[12] |
|
PlusIce PCM A26 |
EPS Ltd. |
Organic solutions |
26 |
150 |
790 |
2.22 |
0.21 |
[12] |
|
Climsel C21 |
Climator |
Hydrated Salt |
21 |
112 |
1380 |
3.6 |
0.5-0.7 |
[13] |
|
Climsel C24 |
Climator |
Hydrated Salt |
24 |
151 |
1380 |
3.6 |
0.5-0.7 |
[14] |
|
Climsel C28 |
Climator |
Hydrated Salt |
28 |
162 |
1380 |
3.6 |
0.7 |
[15] |
|
PCM Latest TM 25T |
TEAP |
Hydrated Salt |
25 |
175 |
1480 |
2 |
1 |
[16] |
BASF offers Micronalw PCM, microencapsulated PCM. The Micronalw PCM is suitable for mixing in fluid substances (paints or adhesives) or more densely in powder form, suitable for inclusion in construction mixes to produce ‘Melting’ dry walls. Micronalw PCM targets building applications offering products with a range of melting temperatures in the human comfort region, 21–26°C. Further manufacturers include Teap, Cristopia, Climator, Mitsubishi Chemical, Doerken and Merck. Of these manufactures, the melting temperatures of PCMs offered range from -50 to 118°C [7].
Building as a thermodynamic system
In a sustainable approach, buildings should be designed to ensure thermal comfort of occupants during the whole year, with a minimum auxiliary energy for heating and cooling. If the storage and insulation properties of the building envelope have a suitable role in the delay and decay of outdoor temperature fluctuation, the indoor air temperature could stay in a comfortable range without heating and/or cooling [17].
The building is a quite complex thermodynamic system, submitted to internal and external solicitations, and a passive ideal energy conservation building is difficult to attain. Many external and internal factors could influence the indoor air temperature fluctuation. External solicitations are due to the local outdoor climatic conditions mainly the air temperature, the wind speed and the solar radiation. Internal solicitations come from internal loads and internal heat source intensity. For specified climatic conditions, air exchange rate, room size, wall thickness and occupation rate and activity, the indoor air temperature is closely related to building’s envelope material properties, mainly their thermal resistance and heat capacity. Therefore, improving the thermal performance of building’s envelope is crucial to reduce the energy consumption for heating and cooling. The thermal energy storage in the building’s envelope, or even in the partition elements, is a key target to attain more energy efficient buildings and PCMs could play an important role in this field [6].
For living conditions to be comfortable, regardless of season, the interior temperature of a building needs to be maintained at a certain level, individually adjusted according to its inhabitants’ tastes, on average from 15 to 25°C. In winter, however, in the absence of its inhabitants, it is sufficient for this temperature to be maintained at a minimum of 5°C [18].
In figure 4 the building is sketched as a thermodynamic system which presents the balance of sources and types of energy from a building, where with yellow are marked the streams of energy reaching the building from the outside (solar and ground energy) are with; with red are marked the sources and streams of thermal energy produced inside the building, generated by house appliances and by its inhabitants; and with blue are marked the stream of energy lost from the building. The building’s envelope (wall, windows, floor, roof, and chimney) is the boundary of the thermodynamic system. The balance between the energy production within the building, the energy consumption and the energy storage during the operational phase of the building is influenced by the internal and external loads, and also by the thermal resistance and heat capacity of the envelope.
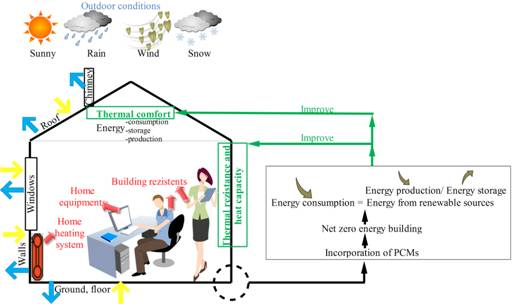
Figure 4. Thermodynamic system which presents the balance of sources and types of energy from a building
The incorporation of PCMs in construction elements enhance the energy storage capacity of the building, helping to reduce the energy consumption for heating and cooling in a passive and sustainable way, and lower energy production is required to attain the energy balance. Hence, PCMs could help attaining a net zero energy building more easily [9].
The main property of PCMs is the storage of heat energy in a latent form, leading to greater heat storage capacity per unit volume than that of conventional building materials. When the ambient temperature rises, the chemical bonds of the material will break up whereby the material will change from solid to liquid. This phase change is an endothermic process and as a result will absorb heat. As the ambient temperature drops again, the PCM will return to the solid state and give off the absorbed heat. This cycle stabilises the interior temperature, cuts off-peak cooling loads and decreases heating loads, not by affecting the thermal resistance of the building envelope but by influencing the (surface) temperatures [10].
PCMs are characterized by a specific phase change (melting and solidifying) occurring at a temperature value or range due to latent heat. When these materials are implemented in building components (e.g. walls), they attain an improved ability to effectively store the solar energy that enters a room in the daytime, leading to the establishment of cooler conditions in internal spaces. Conversely, when temperatures decrease and become lower than the PCM’s characteristic melting point (usually at night), the material solidifies and the previously absorbed heat (latent heat) is released, supporting the maintenance of higher indoor temperatures [20]. The working principle of PCM for buildings consists of following two modes of operation:
Ø Charging process (solidification of PCM): Charging process is carried out during night time when ambient temperature is lower compared to room temperature. The cool ambient air flows through storage unit and takes away heat from liquid PCM which starts solidifying at certain constant temperature. Charging process continues until the ambient temperature is lower enough than the melting/solidification temperature of PCM [4, 22]. The solidification process is dominated by conduction. During solidification natural convection exists only in the beginning and as the time goes the effect of natural convection becomes almost zero as compared to the effect of conduction [23-24].
Ø Discharging process (cooling of air, melting of PCM): Cold stored in PCM is discharged when room temperature rises above the comfort limit. Hot air which is to be cooled passes through the PCM storage unit and PCM (which is in solid state after charging operation) absorbs heat from the air. The air thus cooled to comfort temperature from the storage is delivered to the living space. PCM absorbing heat from air, starts converting from solid to liquid phase at certain constant temperature [4,22]. During melting, heat is transferred to the PCM first by conduction and later by natural convection. This is because, the solid region moves away from the heat transfer surface and the thickness of the liquid region increases near the heat transfer surface. Since thermal conductivity of liquid PCM is less than that of solid PCM, the heat transfer by conduction almost becomes negligible as the melting process continues. The further melting is mostly by natural convection due to the density gradient that exists within the liquid PCM [21].
Incorporation of PCMs into construction materials and elements
The PCM can be incorporated in construction materials and elements by direct incorporation, immersion or encapsulation.
Ø Direct incorporation it is the simplest method in which liquid or powdered PCMs are directly added to building materials such as gypsum, concrete or plaster during production [22]. Direct incorporation may be the most economical method, because very little additional process equipment is required [23]. Direct incorporation can have leakage issues allowing the PCM to flow out of the material system or incompatibility issues with the system itself such as alkaline sensitive PCMs degrading in high alkalinity environments [19].
Ø Immersion processes impregnate a porous construction material such as gypsum, brick or concrete blocks with PCM such that the pores adsorb the PCM in its liquid via capillarity forces [19]. While some researchers pointed out this method may have a leakage problem which is not good for long-term use. Immersion and direct incorporation have different operation processes, but they both incorporate PCMs directly in conventional construction materials [22]. Immersion and direct incorporation can have leakage issues allowing the PCM to flow out of the material system or incompatibility issues with the system itself such as alkaline sensitive PCMs degrading in high alkalinity environments [19].
Ø Encapsulation is another option for adding PCM to construction materials. This methodology entails the encapsulation of PCM in polymer shell usually done by the manufacture or into other materials that could then be incorporated into the building material [19]. This methodology is proposed to reduce incompatibility issues along with leakage as the PCM is encapsulated and prevented from direct contact with the construction material [19]. Encapsulation of PCM occurs before incorporation into the building element, avoiding some of the issues associated with immersion and direct incorporation [19]. The main advantages of PCM encapsulation are providing large heat transfer area, reduction of the PCM reactivity towards the outside environment and controlling the changes in volume of the storage materials as phase change occurs [24].
Microencapsulation is a technology in which PCM particles are enclosed in a thin, sealed and high molecular weight polymeric film maintaining the shape and preventing PCM from leakage during the phase change process [22]. The coated particles can then be incorporated in any matrix that is compatible with the encapsulating film. It follows that the film must be compatible with both the PCM and the matrix [24]. It is much easier and more economic to incorporate the microencapsulated PCMs into construction materials [22]. The advantages for microencapsulation are: improves heat transfer to the surrounding through its large surface to volume ratio and improves cycling stability since phase separation is restricted to microscopic distances [24]. Figure 5 shows a schematic view of the PCM microcapsules integrated into the interior plaster.
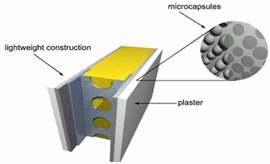
Figure 5. Schematic view of the PCM microcapsules integrated into the interior plaster [25]
Macro encapsulation represents the encapsulation in containers usually larger than 1 cm in diameter. Macro encapsulation comprises the inclusion of PCM in some form of package such as tubes, pouches, spheres, panels or other receptacle. These containers can serve directly as heat exchangers or they can be incorporated in building products. Macro encapsulation is the most common form of encapsulation [24]. With macro encapsulated PCMs, the leakage problem can be avoided and the function of the construction structure can be less affected. It has the disadvantages of poor thermal conductivity, tendency of solidification at the edges and complicated integration to the building materials [22]. Besides holding the liquid PCM and preventing changes of its composition through contact with the environment, macro encapsulation also: improves material compatibility with the surrounding, through building a barrier; improves handling of the PCM in a production; reduces external volume changes, which is usually also a positive effect for an application [24]. Figure 6 shows the CSM panel from RUBITHERMÒ containing the PCM.

Figure 6. CSM panel containing the PCM [26]
This kind of PCM panels, called CSM modules, was made from aluminium with an efficient anti-corrosion coating [22] and can be filled with any type of PCMs providing maximum flexibility to the user, depending on intended use [26].
Conclusions
The review shows that with suitable PCM incorporation in the buildings, it can be an efficient solution to decrease energy use to heat and cool buildings. A small amount of PCMs can raises thermal inertia substantially without increasing the mass of the structure.
References
1. Castellon C., Nogues M., Roca J., Medrano M., Cabeza L.F. (online), Microencapsupated phase change materials (PCMs) for building applications, Available at: http://intraweb.stockton.edu/eyos/energy_studies/content/docs/final_paper s/14b-1.pdf, (accessed 10/06/2014).
2. Fang Y. (online), A comprehensive study of Phase Change Materials (PCMs) for building wall applications, PhD. Thesis submitted to the graduate degree program in Civil engineering and the graduate faculty of the university of Kansas in partial fulfilment of the requirements for the degree of Doctor of Philosophy, 2009, Available at: http://kuscholarworks.ku.edu/dspace/handle/1808/5537, (accessed 10/06/2014).
3. Fang X., Zhang Z., Chen Z. (online), Study on preparation of montmorillonite-based composite phase change materials and their applications in thermal storage building materials, Energy conversion and management, 2008, 49, p.718-723, Available at: http://www.sciencedirect.com/science/article/pii/S0196890407002518, (accessed 10/06/2014).
4. Waqas A., Ud Din Z., (online), Phase change material (PCM) storage for free cooling of buildings - A review, Renewable and sustainable energy reviews, 2013, 18, p.607-625, Available at: http://www.sciencedirect.com/science/article/pii/S136403211200576X, (accessed 01/07/2014).
5. Memon S.A. (online), Phase change materials integrated in building walls: A stat of the art review, Renewable and sustainable energy reviews, 2014, 31, p.870-906, Available at: http://www.sciencedirect.com/science/article/pii/S1364032113008563, (accessed 02/07/2014).
6. Maldonado P.A. (online), Application of passive thermal energy storage in buildings using PCM and awnings, Ph.D Thesis, Universitat de Lleida, 2011, Available at: http://www.tdx.cat/bitstream/handle/10803/32001/Tpam1de1.pdf?sequence=1, (accessed 20/06/2014).
7. Whiffen T.R., Riffat S.B. (online), A review of PCM technology for thermal energy storage in the built environment: Part I, International journal of low-carbon technologies advance acces, 2012, 0, p.1-12, Available at: http://ijlct.oxfordjournals.org/ content/early/2012/05/30/ijlct.cts021, (accessed 04/07/2014).
8. Koschenz M., Lehmann B. (online), Development of a thermally activated ceiling panel with PCM for application in lightweight and retrofitted buildings, Energy and buildings, 2004, 36(6), p.567-578, Available at: http://www.sciencedirect.com/science/article/pii/ S0378778804000702, (accessed 04/07/2014).
9. Soares N., Costa J.J., Gaspar A.R., Santos P. (online), Review of passive PCM latent heat thermal energy storage systems towards buildings `energy efficiency, Energy and buildings, 2013, 59, p.82-103, Available at: http://www.sciencedirect.com/science/ article/pii/S0378778813000157, (accessed 10/06/2014).
10. Baetens R., Jelle B.P., Gustavsen A. (online), Phase change materials for building applications: A state-of-the-art review, Energy and buildings, 2010, 42, p.1361-1368, Available at: http://www.sciencedirect.com/science/article/pii/S0378778810001180, (accessed 01/07/2014).
11. Rubitherm (online), Available at: http://www.rubitherm.com/english/index.htm, (accessed 04/07/2014).
12. EPS Ltd (online), Available at: http://www.pcmproducts.net/files/PlusICE%20Range-2013.pdf, (accessed 04/07/2014).
13. Climsel C21 (online), Available at: http://www.climator.com/files/products/climsel-c21.pdf, (accessed 04/07/2014).
14. Climsel C24 (online), Available at: http://www.climator.com/files/products/climsel-c24.pdf, (accessed 04/07/2014).
15. Climsel C28 (online), Available at: http://www.climator.com/files/products/climsel-c28.pdf, (accessed 04/07/2014).
16. TEAP (online), Available at: http://www.teappcm.com/products.htm, (accessed 04/07/2014).
17. Zhang Y., Lin K., Zhang Q., Di H. (online), Ideal thermophysical properties for free-cooling (or heating) buildings with constant thermal physical property material, Energy and buildings, 2006, 38(10), p.1164-1170, Available at: http://www.sciencedirect.com/ science/article/pii/S0378778806000387, (accessed 10/06/2014).
18. Lewandowski W.M., Lewandowska-Iwaniak W. (online), The external walls of a passive building: A classification and description of their thermal and optical properties, Energy and buildings, 2014, 69, p. 93-102, Available at: http://www.sciencedirect.com/science/article/pii/S0378778813006671, (accessed 01/07/2014).
19. Sharma B. (online), Incorporation of Phase Change Materials into Cementitious Systems, Master thesis, 2013, Available at: http://repository.asu.edu/attachments/125 776/content/Sharma_asu_0010N_13319.pdf, (accessed 06/07/2014).
20. Mandilaras I., Stamatiadou M., Katsourinis D., Zannis G., Founti M. (online), Experimental thermal characterization of a Mediterranean residential building with PCM gypsum board walls, Building and environment, 2013, 61, p.93-103, Available at: http://www.sciencedirect.com/science/article/pii/S0360132312003290, (accessed 01/07/2014).
21. Jegadheeswaran S., Pohekar S.D. (online), Performance enhancements in latent heat thermal storage system: A review, Renewable and sustainable energy reviews, 2009, 13, p.2225-2244, Available at: http://www.sciencedirect.com/science/article/pii/ S1364032109001221, (accessed 01/07/2014).
22. Zhou D., Zhao C.Y., Tian Y. (online), Review on thermal energy storage with phase change materials (PCMs) in building applications, Applied energy, 2012, 92, p.593-605, Available at: http://www.sciencedirect.com/science/article/pii/S0306261911005216, (accessed 05/07/2014).
23. Bajare D., Kazjonovs J., Korjakins A. (online), The thermal characteristics of gypsum boards with phase change materials (PCMs), Proceedings of the 8th International scientific and practical conference, 2011, II, p.132-138, Available at: http://zdb.ru.lv/conferences/3/VTR8_II_132.pdf, (accessed 06/07/2014).
24. Cabeza L.F., Castell A., Barreneche C., deGacia A., Fernandez A.I. (online), Materials used as PCM in thermal energy storage in buildings: A review, Renewable and sustainable energy reviews, 2011, 15(3), p.1675-1695 Available at: http://www.sciencedirect.com/ science/article/pii/S1364032110003874, (accessed 06/07/2014).
25. Tyagy V.V., Kaushik S.C., Tyagi S.K., Akiyama T., (online), Development of phase change materials based microencapsulated technology for buildings: A review, Renewable and sustainable energy reviews, 2011, 15, p.1373-1391, Available at: http://www.sciencedirect.com/science/article/pii/S1364032110003461, (accessed 06/07/2014).
26. Rubitherm (online), Available at: http://www.rubitherm.de/english/, (accessed 06/07/2014).