Assessing the effect of copper additions on the corrosion behaviour of grey cast iron
Saliu Ojo SEIDU1, Seun Samuel OWOEYE2*, Helen Tola OWOYEMI3
1Department of Metallurgical and
Material Engineering,
2Department of Glass and Ceramics
Technology, Federal Polytechnic, Ado-Ekiti, P.M.B.
5351,
3Department of Petroleum and Mineral
Resources Engineering, Federal Polytechnic, Ado- Ekiti,
P.M.B. 5351,
Emails: seidu2@yahoo.com; *owoeyeseun@yahoo.com; howoyemi11@yahoo.com
*Corresponding author, phone: +2348064461915
Abstract
In this research work, the effect of copper additions on the corrosion behaviour of grey cast iron in 3.5 wt% NaCl, 0.3M H2SO4, and 0.1M NaOH respectively was investigated. Grey cast iron samples containing 3.0%, 2.5%, 2.0%, and 1.5% weight percent of copper were produced. The corrosion behaviour of the grey cast iron samples produced were assessed using mass loss and corrosion rate measurements according to America Society for Testing and Materials standard (ASTM) procedures in salt water, basic, and acidic environments. The results reveal that the samples containing 2.0% and 1.5% weight percent of copper show an excellent corrosion resistance while samples containing 3.0% and 2.5% weight percent of copper show good corrosion behaviour all in salt water and basic environments but poorly in acidic environment.
Keywords
Grey cast iron; Corrosion rate; Mass loss; Copper; Salt water; Basic environment; Acidic environment
Introduction
Grey cast iron is one of the most widely used metallic materials, because of its good castability, excellent machinability, relatively low cost and unique properties [1, 2]. Due to the recent rapid increase in demand for high performance of equipment and machines, a great deal of research has been on how to improve the mechanical properties of the grey cast iron during performance [3]. One of the important methods used is to produce a metal matrix composite material reinforced with a higher strength material. Inoculation with hardenability elements has also been used to improve the property of this cast iron [2].
Grey cast iron is characterized by presence of a large portion of its carbon in the form of graphite flakes. Although grey cast iron is often defined as steel containing graphite, its properties are far different from those of steels [4].
The predominant deterioration mechanism on the exterior of grey cast iron pipes are electrochemical corrosion with damage occurring in form of pits. The deterioration of grey cast iron is often disguised by the presence of graphitization [5]. Alloying elements have been discovered to play a major role in the susceptibility of cast iron to corrosion attack. Alloy additions to grey cast iron have been found to improve the corrosion resistance by modifying the matrix since a fine pearlite is preferred to a ferritic structure [6]. Alloy additions have also been found to be advantageous in alkaline and acid environments where plain iron possess little resistance to corrosive attack [7]. One of the most important alloying element used to improve corrosion resistance of cast iron is silicon which is generally considered as an alloying element when exceeds 3%. Silicon offers some increase in corrosion resistance when use between 3% and 14% [8]. Carbon when use at considerable amount has also been found to enhance corrosion resistance in cast iron generally as a result of the free graphite resulting into an insoluble graphite layer of corrosion product which are dense, adherent, forming a barrier against further corrosion [9]. Nickel, chromium, and copper have also been observed to increase corrosion resistance of grey cast iron and low alloy steel in cocoa liquor [10]. A copper addition of 0.25 to 1% was observed to increase corrosion resistance of steel in aqueous environment in conjunction with nickel and chromium present in the composition [10].
Various research works have been performed to determine the susceptibility of cast iron generally in aqueous solutions [11]. Basically, susceptibility of metals to corrosion attack in aqueous solutions (salts, acids, and alkali) is dependent on the dissolved salts and oxygen [12].
This present work focuses on assessing the effect of copper addition on the corrosion behaviour of grey cast iron in salt water, basic, and acidic environment.
Material and method
The corrosion rate (C.R) and mass loss (m.l) used for evaluating the corrosion behavior in this research work are determined from weight loss measurement in accordance with ASTM G31 as stated by [13].
C.R = KW/ρAt (1)
where C.R is the corrosion rate (mpy), K is a constant equals to 87500, W is the weight loss (g), ρ is the density (g/cm3), A is the area (cm2) and t is the exposure time (yr).
W = Wi - Wf (2)
where W is the weight loss (g), Wi represents initial weight (g), and Wf is the final weight (g)
m.l = CW/A (3)
where m.l is the mass loss (g/cm3), CW is the cumulative weight (g), and A is the surface area (cm2).
Equations 1, 2, and 3 show relationships between weight loss, mass loss, and corrosion rate.
Grey cast iron production
A 40kg charge materials comprising of cast iron scraps, graphite (Carbon), and ferro-alloys were accurately composed and weighed, which was later charged into the 100kg capacity rotary furnace after initial pre-heating of the furnace The charge was then heated to about 1500°C and the melt held at this temperature for about 15mins for homogenization. The melt was then tapped inside ladles containing varying inclusion of copper which were then allowed to cool before de-moulding.
Sample preparation
Four different samples of as-cast grey iron containing 3.0%, 2.5%, 2.0%, 1.5% weight percent were utilized in this present study. The test pieces were cut into suitable sizes hand-able enough without machine mounting during grinding and polishing. These test pieces were true representation of the mass of the samples.
Immersion testing
The corrosion tests were carried out in the already prepared environments, namely: 3.5wt% NaCl of pH 7.4, 0.3M H2SO4 of pH 0.85, and 0.1M NaOH of pH 12.7 solutions following standard procedures. The test pieces for the test were cut into 20x20x5mm. This means 12 test pieces were obtained with 3 test pieces from each samples 1, 2, 3, and 4 to be immersed inside the three environments prepared. The test pieces surfaces were afterward mechanically polished using an emery papers commencing from 120grit down to 640 grit size. The test pieces were de-greased using acetone and then rinsed in de-ionized water before immersion into the already prepared environments of 3.5wt% NaCl, 0.3M H2SO4, and 0.1M NaOH, which were all exposed to atmospheric air. The solution-to-specimen surface area ratio was about 150ml/cm2. The results of the corrosion tests were evaluated by mass loss, corrosion rate measurements, and potential difference on two day intervals. The samples were exposed in the acidic, basic and alkaline environments for the period of 40 days. Mass loss (mg/cm2) for each sample was evaluated by dividing the cumulative weight loss (i.e. m.l. = CW/A) by its total surface area which is in accordance with ASTM standard recommended practice [13]. Corrosion rate for each sample was evaluated from the weight loss measurements following standard procedures.
Results and discussion
Figure 1 represent the corrosion rate against exposure time for the as-cast samples 1, 2, 3, and 4 containing 3.0%, 2.5%, 2.0%, and 1.5% (weight percent) respectively in H2SO4.
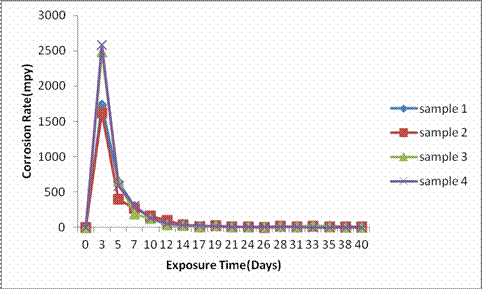
Figure 1. Graph of corrosion rate of samples in 0.3M H2SO4
It was observed that all the as-cast samples exhibited the same completely unacceptable corrosion rate trend as their values were above 200mpy [14, 15] for the first five days of immersion for all the samples, however, there was a drastic drop in the corrosion rate after 5 days at values a little <500mpy while passivation was observed from the 12th day of immersion and which was maintained for the rest of the immersion period. However, all the as-cast samples can be considered poor in this environment.
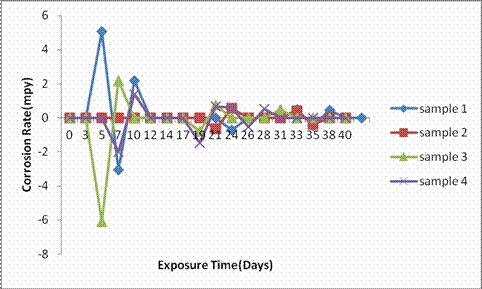
Figure 2. Graph of corrosion rate of samples in 0.1M NaOH
Figure 2 indicates the corrosion rate against exposure time of the as-cast samples 1, 2, 3, and 4 in alkali environment. It was observed that sample 2 shows an outstanding corrosion rate of <1mpy all through the immersion period indicating an outstanding corrosion resistance according to Mehra and Soni, 2002. While samples 1, 3 and 4 displayed excellent corrosion rate of 5mpy, 2mpy, and 1mpy respectively [5], an outstanding corrosion rate of <1mpy was only observed in all the as-cast samples 1, 3, and 4 after 10 days of immersion and which were maintained for the rest of the immersion period [15, 14]. The excellent corrosion rate observed by all the as-cast samples in alkali medium could be as a result of the strong protective layer formed due to the presence of copper in combination with nickel, silicon, and chromium present in their composition.
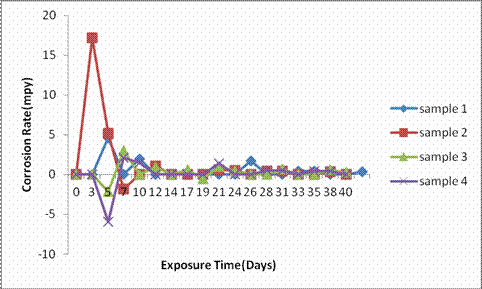
Figure 3. Graph of corrosion rate of samples in 3.5wt% NaCl
Figure 3 indicates the corrosion rate against exposure time of the as-cast samples 1, 2, 3, and 4 in the marine (3.5wt. % NaCl) environment. It was observed from the plot that sample 2 exhibited intense corrosion rate of 17.2mpy within the first 3 days; this value is considered good corrosion resistance according to [14] and [15] range of 5-20mpy for good corrosion resistance behaviour. A drastic drop to <1mpy after 5days of immersion was observed in the sample 1 and was maintained all through the immersion period indicating outstanding corrosion resistance according to [14] and [15]. Samples 1, 3, and 4 showed an excellent corrosion resistance with corrosion rate falling within the range 1-5mpy in the environment [14, 15]. This is a clear indication that these as-cast samples 1, 2, 3, and 4 can be proven to be a good metal for use in the marine environment.
Figures 4, 5 and 6 indicate the plot of mass loss against exposure time of the as-cast samples 1, 2, 3, and 4 in H2SO4, alkali, and salt environments.
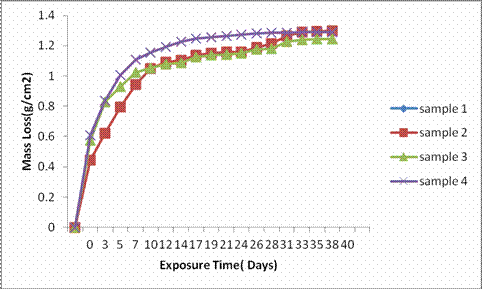
Figure 4. Graph of mass loss of samples in 0.3M H2SO4
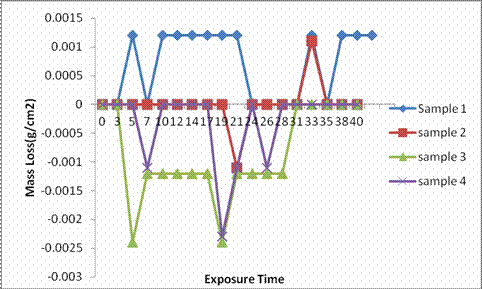
Figure 5. Graph of mass loss of samples in 0.1M NaOH
It was observed that the mass loss was predominant in all the as-cast gray cast iron samples with respect to exposure time in H2SO4 i.e. mass loss increases with increase in exposure time and this continue throughout the exposure time. This clearly indicates that corrosion susceptibility increases with increase in exposure time and thereby indicates that the as-cast samples cannot be use in such environment. Samples 3 and 4 shows an outstanding mass loss trend with their values far below zero in alkali environment, this indicate high resistance due to weight gained as a result of passivation all through the exposure period which also justify their excellent corrosion rate in the same environment. Sample 2 also shows an outstanding mass loss trend due to weight gained as a result of passive film being formed but which was later broken after 31st day of immersion which is also in agreement with its corrosion rate trend in the same environment. Mass loss was predominant in sample 1, however, drastic drop in mass loss was observed at one point or other throughout the immersion period.
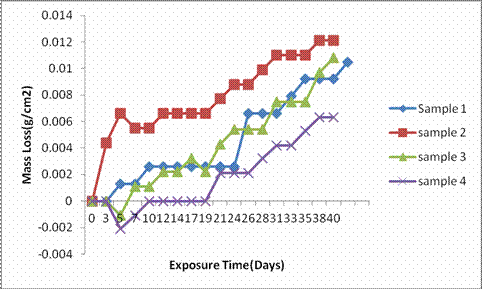
Figure 6. Graph of mass loss of samples in 3.5wt% NaCl
It was observed from Figure 6 that all the as-cast samples exhibit similar mass loss trend in marine environment. However, their mass loss values fall within the range 0 – ≤ 0.01g/cm2 with sample 4 showing the least mass loss of 0.004g/cm2 while mass loss was only predominant in sample 2 up to 0.01g/cm2 which indicates mass loss increases with exposure time in the sample. All the as-cast samples can therefore be regarded successful in the marine environment.
Conclusion
From the results obtained in this present work, it can be seen that samples containing copper content of 2.0% and 1.5% weight percent exhibited good corrosion resistance in alkali and marine environment except in acidic environment in which all the samples performed poorly. It can therefore be concluded within the limit of this research that copper when used at low weight percent (1.0-2.0%) enhances corrosion resistance in grey cast iron in combination with elements such as silicon, nickel, and chromium present in its composition.
References
1. Charles F. W., Iron casting handbook, Iron Casting Society, Inc., 1981.
2. Xu W., Ferry M., Wang Y., Influence of alloying elements on as-cast microstructure and strength of gray iron, Materials Science and Engineering A 390, 2005, p. 326-333.
3. Edalati K., Akhlaghi F., Nili-Ahmadabadi M., Influence of SiC and FeSi addition on the characteristics of gray cast iron melts poured at different temperatures, Journal of Minerals and Materials Characterization and Engineering, 2005, 160(2), p.183-187.
4.
Rajput R. K., Engineering materials and
metallurgy, 1st ed. S. Chand & Co.
5. Sami A.A., Safaa M.H., Ductile and gray cast iron deterioration with time in various NaCl salt concentrations, J. Engr. & Tech., 2008, 26(1), p.1-15.
6.
Gray Iron Founders’
Society, Gray cast iron handbook,
7. Maxwell H.L., Use of cast iron in the chemical industry, Chem. Engr. Congress of the World Power Conference, 1936.
8. Rana A. M., Khan A., Amjad S., Abbas T., Microstructural evaluation in heat-treated cast iron, Journal of Research (Science), Bahauddin Zakariya Univeristy, Multan, Pakistan, 2001, 12(1), p. 65-71.
9. Fredric T., High temperature corrosion of cast iron and steels, Chalmers University of Technology, 2004.
10. Olawale J. O., Jamiu K. O., Abdulkarim B. R., Evaluation of corrosion behaviour of grey cast iron and low alloy steel in cocoa liquor and well water, Journal of Minerals and Materials Characterization and Engineering, 2013, 1, p. 44-48.
11. Osarolube E., Owate I. O., Oforka N. C., Corrosion behaviour of mild and high carbon steels in various acidic media, Scientific Research and Essay, 2008, 33(6), p. 224-228.
12.
Ibrahim S. L., Corrosion of boiler steel
pipes under given operating conditions in the presence and absence of
some O2 scavengers,
13. Bodurin, M. O., Alaneme, K. K., Olusegun, S. J., Influence of Thermomechanical Processing on the Corrosion Behaviour of Aluminium(6063)-SiCp Composites in NaCl and H2SO4 Environment, Sci. J. UBU, 2011, 2(2), p. 17-25.
14. Fontana M. G., Corrosion engineering, McGraw- Hill Publications, 1986, 3rd Ed., p.172.
15. Mehra R., Soni A., Cast iron deterioration with time in various aqueous salt solutions, Bull. Mater. Sci., 2002, 25(1), p.53-58.