Study of carbon and silicon loss through oxidation in cast iron base metal using rotary furnace for melting
Sylvester Olanrewaju OMOLE1*, Raymond Taiwo OLUYORI2
1Metallurgical
and Materials Engineering Dept;
2Metallurgical
Engineering Dept; School of Engineering,
E-mails: *sylvesteromole@yahoo.com, taiwo_oluyori@yahoo.com
*Corresponding author, phone: +2347033089934, +2348053043899
Abstract
The projection of loss of carbon and silicon through oxidation is uncertain phenomenon depending on the furnace used for melting, which affect the carbon equivalent value (CEV) of cast iron produced. CEV enhances the fluidity of molten metal as well as having great effects on the mechanical properties of cast products. Study on the way elemental loss takes place during melting with rotary furnace will give idea of approach to minimize the loss. Therefore, the aim of this work is to study the magnitude of the elemental loss with rotary furnace and means to minimize the loss. 60kg of grey cast iron scrap was charged into rotary furnace of 100kg capacity after preheating the furnace for 40 minutes. Graphite and ferrosilicon was added to the charge in order to obtain a theoretical composition of not less than 4.0% carbon and 2.0% silicon. Charges in the furnace were heated to obtain molten metal which was tapped at 1400°C. Tapping was done for casting at three different times. The castings solidified in sand mould and allowed to cool to room temperature in the mould. Castings were denoted as sample 1, 2 and 3. Final compositions of each casting were analyzed with optical light emission spectrometer. Sample 1 has 2.95% carbon and 1.82% silicon. Sample 2 has 2.88% carbon and 1.70% silicon and sample 3 has 2.75% carbon and 1.63% silicon.
Keywords
Elemental loss; Optical light emission spectrometer; Ferro-silicon; Carbon equivalent value; Grey cast iron; Ductile Cast Iron; Rotary furnace
Introduction
Conventional furnaces for producing ductile iron are induction furnaces of different types, electric arc furnace and cupola furnace. Rotary furnace was developed and or modified for the production of cast iron in order to complement, or in the absence of the conventional furnaces. Rotary furnaces have cylindrical barrel which revolve completely at the rate of about one revolution per minute [1]. Their capacity ranges from few kilograms to several tonnes, there is a burner positioned at one end of the barrel; rotary furnaces are fired by oil or pulverized coal. Combination of fuel and air generates high temperature flame which melts and superheats the charge in the barrel.
Foundry provides components and raw
materials to other industries, for this reason it is the mother of all other
industries. Foundry relies basically on imported raw materials
and processing equipment in the developing nations such as
The family of ductile cast iron covers a wide range of mechanical properties, replacing successfully both cast and forged steel and malleable cast irons in many applications (such as wheels, gears, crankshaft in cars and trucks) [7]. Matrix plays a key role in determining the overall properties combination and allowing obtaining high ductility values (up to more than 18% rupture elongation) and high strength (up to 850MPa and, considering austempered ductile iron up to 1600MPa) with a good wear resistance [7]. Matrix names are usually used to designate ductile cast iron types. Production of ductile iron is achieved by adding magnesium into suitably treated molten iron and then the graphite precipitates in the form of spheroids [8]. It is not easy to add magnesium to liquid iron; magnesium boils at a low temperature (1090°C), so there is a violent reaction due to the high vapor pressure of Mg at the treatment temperature causing violent agitation of the liquid iron and considerable loss of Mg in vapor form [9].
Induction and electric arc furnaces are not
adequately available in some developing countries especially
The aim of this research was to study the functionality of rotary furnace in terms of oxidation of the major elements present in cast iron base metal during melting. Many users of rotary furnace have projected only about 10% elemental losses in rotary furnace which was discovered to be more than 10 % loss with this research. The loss in major elements has made composition control difficult during the use of rotary furnace.
Materials and method
Melting with rotary furnace
60kg of grey cast iron scrap of known chemical composition was charged into the rotary furnace along with 0.5kg graphite and 0.2kg ferrosilicon, after preheating the furnace between 30 to 40 minutes. The charge was prepared to have a theoretical carbon and silicon composition of not less than 4.0% and 2.0% respectively, taking into consideration only 10% elemental loss.
The furnace was heated with diesel oil as fuel; air and the oil are injected into the furnace for burning. The furnace was rotated periodically after every 20 minutes of firing before the charges begin to melt. When it has melted, the furnace is rotated continuously for adequate mixing before pouring. The molten metal was tapped at a temperature of 1400°C.
Casting of the metal
Mould cast was prepared from silica sand and bentonite (clay) forming green sand. Pattern used was a cylindrical wooden pattern of diameter 25mm from which test sample was obtained. A refractory lined ladle was used to collect molten metal from the furnace for casting. Three different cast samples were made from three tapping at about 10 to 15 minutes interval between each tapping. The castings were allowed to solidify in the mould to room temperature before removed in the mould, fettled and cleaned.
Composition analysis
A sample was cut from each casting (as sample 1, 2 and 3 in the order in which tapping was made for casting). Each sample was grinded with emery paper of 400 and 800 grits. The grinding was done to obtain smooth and flat surfaces on the samples. Optical light emission spectroscopy was used to obtain average composition in each sample after making spark on three different portions on the samples.
Table 1. Composition of C, Si and P in cast metal (Sample1, 2 and 3)
|
Sample |
% C |
% Si |
% P |
|
1 |
2.95 |
1.82 |
0.073 |
|
2 |
2.88 |
1.70 |
0.068 |
|
3 |
2.75 |
1.63 |
0.070 |
It is recommended that higher quantity of graphite should be added into any charge prepared as it will help to minimize the rate of loss of carbon, even if the carbon yield into the melt will be small. Inoculation is highly recommended as it will make up for the loss of silicon in the furnace. In totality excessive heating of the melt especially when the metal has attained tapping temperature should be minimized or possibly avoided.
Results and discussion
Carbon equivalent value calculation for the iron produced
CEV = %C + (%Si + %P)/3 [5, 6].
Theoretical CEV for the prepared melt = 4.0 + (2.0 + 0.088)/3 = 4.67
CEV for sample 1 = 2.95 + (1.82 + 0.073)/3 = 3.58
CEV for sample 2 = 2.88 + (1.70 + 0.068)/3 = 3.47
CEV for sample 3 = 2.75 + (1.63 + 0.070)/3 = 3.32
Based on the result above in Table 1, the resulting composition obtained from the test shows that carbon and silicon contents have been oxidized compared to the expected value of 4.0% carbon and 2.0% silicon. Figure 1 is a chart which shows the various percentage losses in carbon and silicon in each sample as 26.25% C, 9.0% Si for sample 1, 28.0% C, 15.0% Si for sample 2 and 31.25% C, 18.5% Si for sample 3.
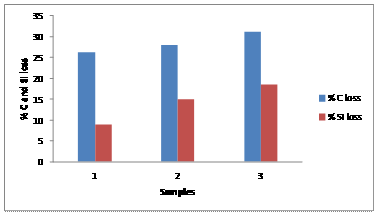
Figure 1. Comparison of C and Si loss in the samples cast
The losses in composition by oxidation were calculated from the differences in the values of carbon and silicon prepared in the charge melted to the value obtained in the casting after test. From Figure 1, it is shown that the rate of losses in the elements discussed increased as the time of firing the melt in the furnace increases before tapping. The oxidation can be said to be due to the mode of operation of rotary furnace in which the fuel, charges and the products of combustion are all in contact. Carbon oxidized more than the silicon despite the fact that graphite as recarburizer was added into the charge in the furnace. This observation is because the graphite which is a source of carbon easily supports combustion, this is why coke can be used as fuel for melting in furnaces [10]. Therefore the rate of oxidation of carbon in the melt is higher. Rate of elemental loss in rotary furnace is therefore a function of the period (time) the molten metal is left in the furnace yet to be tapped, after attaining the required tapping temperature. Higher time results in higher loss of elements. More time given to the melt during super heating in the furnace and time to complete the discharge of the molten metal in the furnace need to be controlled, in order to reduce the percentage elemental loss in the casting.
Conclusion
From the results obtained, elemental loss during processing of material in rotary furnace is a time dependent phenomenon, because sample 3 has the highest loss of carbon and silicon, followed by sample 2 and finally sample 1 has the lowest loss. It has also been clearly shown that using rotary furnace for melting resulted in more than 10 % loss in carbon contrary to the believe of some users of the furnace.
References
1. Agarwal R. L., Banga T. R., Tahil Nanghnani, Foundry engineering, Khanna Publishers India, 1981, pp. 150-280.
2. Oyetunji A., Seidu S. O., Synergistic effects of starch and rubber-latex as core binder for foundry sand cores production, Acta Technica Cornviniencis - Bulletin of Engineering 2012, 4, p. 103-106.
3. Steve H., Metal casting: appropriate technology in the small foundry, Intermediate Technology Publications U.K., 1996, pp. 54-65.
4. Masoud Z., Seyyed M. A. B., Fracture characteristics of austempered spheroidal graphite aluminium cast irons, Journal of Iron and Steel Research International, Science Direct, 2010, 17(2), p. 31-35.
5. Tiedje N. S., Solidification, processing and properties of ductile iron, Materials Science and Technology, 2010, 26(5), p. 505-515.
6. Brown J. R., Foseco
foundry man handbook, Butter worth - Heinemann
7. Dicocco V., Lacoviello F., Cavallini M., Damaging micro mechanisms characterisation of a ferritic ductile cast iron, Journal of Engineering Fracture Mechanics, 2010, 77, p. 2-3.
8. Oyetunji Akinlabi, Omole S. O., Evaluation of ductile iron produced using rotary furnace with variable compositions of magnesium addition, International Journal of Science and Advanced Technology (IJSAT), 2011, 1(9), p. 276-282.
9. Murat B., Seckin I. A., Successive boronizing and austempering for GGG 40 grade ductile iron, Journal of Iron and Steel Research International Science Direct, 2009, 16(2), p. 50-54.
10.
Jain P. L., Principles
of foundry technology, Third edition, Tata
McGraw-Hill Publishing Company Ltd.