Corrosion behaviour of groundnut shell ash and silicon carbide hybrid reinforced Al-Mg-Si alloy matrix composites in 3.5% NaCl and 0.3M H2SO4 solutions
Kenneth Kanayo ALANEME*, Henry Ikechukwu EZE, Michael Oluwatosin BODUNRIN
Department of Metallurgical and Materials Engineering, Federal University of Technology, Akure, PMB 704, Nigeria
E-mail: 1* kalanemek@yahoo.co.uk
* Corresponding author, phone: +2348034228868
Abstract
The corrosion behaviour of Al-Mg-Si alloy based composites reinforced with groundnut shell ash (GSA) and silicon carbide (SiC) was investigated. The aim is to assess the corrosion properties of Al-Mg-Si alloy based hybrid reinforced composites developed using different mix ratios of GSA (a cheaply processed agro waste derivative which served as partial replacement for SiC) and SiC as reinforcing materials. GSA and SiC mixed in weight ratios 0:1, 1:3, 1:1, 3:1, and 1:0 were utilized to prepare 6 and 10 wt% of the reinforcing phase with Al‐Mg‐Si alloy as matrix using two‐step stir casting method. Mass loss and corrosion rate measurement was used to study the corrosion behaviour of the produced composites in 3.5% NaCl and 0.3M H2SO4 solutions. The results show that the Al-Mg-Si alloy based composites containing 6 and 10 wt% GSA and SiC in varied weight ratios were resistant to corrosion in 3.5% NaCl solution. The composites were however more susceptible to corrosion in 0.3M H2SO4 solution (in comparison with the 3.5% NaCl solution). It was noted that the Al-Mg-Si/6 wt% GSA-SiC hybrid composite grades containing GSA and SiC in weight ratio 1:3 and 3:1 respectively exhibited superior corrosion resistance in the 0.3M H2SO4 solution compared to other composites produced for this series. In the case of the Al-Mg-Si/10 wt% GSA-SiC hybrid composite grades, the corrosion resistance was relatively superior for the composites containing a greater weight ratio of GSA (75% and 100%) in 0.3M H2SO4 solution.
Keywords
Al-Mg-Si alloy; Hybrid composites; Corrosion; Groundnut shell ash; Silicon carbide
Introduction
The design of particle reinforced aluminium matrix composites (AMCs) has continued to generate a lot of interest from researchers [1, 2]. Particle reinforced composites have the advantages of low cost of processing, improved isotropic properties and possibility of subjecting the materials to conventional post fabrication processes such as welding and mechanical working [3, 4]. There is currently the innovation of processing industrial and agro wastes as particulates for reinforcing AMCs [5, 6]. The fact that these wastes can be processed to particulates even to nano-size ranges at relatively low cost using simple processes adds to the versatility and attraction of AMCs development using particle reinforcement [7].
AMCs reinforced with synthetic particulates such as alumina and silicon carbide have been applied successfully in the development of components and parts for use in aerospace, marine, defence, sports, and recreational industries [8-10]. This is due largely to their attractive spectrum of properties which include and are not limited to high specific strength, high specific stiffness, excellent wear resistance, light weight and sometimes corrosion resistance [11-13]. Research efforts in recent times have been geared towards cost reduction of AMCs by partial or total replacement of the synthetic particle reinforcements with processed industrial and agro wastes with the intention of still maintaining the performance levels obtainable with the use of only synthetic reinforcements [14, 15]. The effect of varied agro waste materials and parameters such as particle size, weight percent, and mix ratio with synthetic particles on material properties of AMCs are currently under study [16, 17].
Research works are now available in literature reporting the viability of the use of different agro waste ashes (such as bagasse, coconut shell, palm nut shell, rice husk, bamboo leaves, and corn cob among others) for use as reinforcing materials in AMCs [17]. The consideration of ground nut shell as a potential reinforcing material for development of AMCs has hardly been covered in literature. Groundnut shell is an agro waste that is available in large quantity in Nigeria [18, 19]. It is obtained when groundnut seed is removed from the shell. Abdulazeez et al [19] reported that Nigeria is the fourth largest producer of groundnut with a proportion of 4.5% of total world production. In 2010 for example, groundnut was grown on over 25 million hectares worldwide with total production output of 37.64 million metric tons. In Africa, Nigeria and Senegal account for about 45% of total groundnut production [19]. This gives a good indication that a large volume of groundnut is produced in Nigeria. It is regrettable to acknowledge that after extraction of the seeds during post harvest processing, disposal of the groundnut shells possess a major challenge in our environment. This is due to limited secondary applications of the groundnut shell. Efforts have been made to recycle groundnut shells by processing it into ash for use as cement replacement in concrete mixtures due to their pozzolanic characteristics (attributable to their high silica and alumina content) [20, 21]. The use of groundnut shell ash to produce particle reinforcement for AMCs will further add value to efforts in groundnut shell waste management.
The use of particulate reinforced AMCs in marine industries and other companies where they come in contact with acid during pickling and cleaning has necessitated studying the corrosion behaviour of AMCs. Unlike the consistency observed for the mechanical properties of AMCs [22], it is very unlikely to predict the corrosion behaviour of AMCs [23, 24]. There are cases where AMCs are resistant to corrosion and there are cases where they are highly susceptible to corrosion [24]. This unpredictability has been attributed to a lot of factors such as reinforcement particle sizes, matrix alloy, types of particulates, interfacial reaction, processing technique among others [25]. Research articles on the corrosion behaviour of AMCs reinforced with agro waste ash as complement reinforcement are very scanty; although the findings have been very interesting, the few ones that are available are for AMCs developed with the use of bamboo leaf ash and rice husk ash [26]. Alaneme and Olubambi [26] reported that single reinforced AMCs possessed superior corrosion resistance when compared to AMCs reinforced with hybridized alumina and rice husk ash (RHA) particulates in 3.5% NaCl environment. But, it was reported that bamboo leaf ash and alumina reinforced hybrid AMCs exhibited superior corrosion resistance in 3.5% NaCl environment when compared with single reinforced AMC [27 ]. These findings are exemplary of the sort of variation in corrosion characteristics of AMCs. Hence, the need to investigate the corrosion behaviour of newly developed aluminium based composites. Therefore, this research work investigated the corrosion behaviour of AMCs reinforced with silicon carbide and groundnut shell ash. This is aimed to assessing the influence of different mix ratios of GSA (the agro waste derivative serving as partial substitute for SiC) and SiC on the corrosion behaviour of Al-Mg-Si based composites developed using both materials as hybrid reinforcement.
Material and method
Materials
The materials utilized in this research work include Al-Mg-Si alloy (Al 6063), silicon carbide particulates, groundnut shell ash, and magnesium. The aluminium alloy with chemical composition presented in Table 1 served as the matrix while chemically pure silicon carbide and groundnut shell ash were used as the reinforcement. The average particle sizes of the silicon carbide particulates and groundnut shell ash used were 30μm and <50μm respectively. The magnesium procured was used to improve the wettability between the reinforcing particulates and the liquid aluminium alloy during stir casting.
Table 1. Elemental composition of Al-Mg-Si alloy
|
Element |
wt % |
Element |
wt % |
|
Si |
0.4002 |
Sn |
0.0021 |
|
Fe |
0.2201 |
Pb |
0.0011 |
|
Cu |
0.0080 |
Ca |
0.0015 |
|
Mn |
0.0109 |
Cd |
0.0003 |
|
Mg |
0.3961 |
Li |
0.0000 |
|
Cr |
0.0302 |
Na |
0.0009 |
|
Zn |
0.0202 |
V |
0.0027 |
|
Ti |
0.0125 |
Al |
98.88 |
|
Ni |
0.0101 |
|
|
Production of groundnut shell ash
Groundnut shells were obtained from Enugu State located at 6°30’N and 7°30’E in Nigeria. The groundnut shells were burnt in open air in a metallic drum to obtain the ash. The ash from the burning process was allowed to cool and collected after 24 hours from the metallic drum. Thereafter, the ash was conditioned in a heating furnace at a temperature of 650°C for 3 hours as described by Alaneme et al [27]. Sieving of the groundnut shell ash was then performed using a sieve shaker to obtain ashes with mesh size under 50μm. The chemical composition of the groundnut shell ash is presented in Table 2.
Table 2. Composition of groundnut shell ash
|
SiO2 |
Al2O3 |
Fe2O3 |
CaO |
MgO |
TiO2 |
Na2O |
K2O |
P2O3 |
MnO |
SO3 |
*LOI |
|
41.42 |
11.75 |
12.60 |
11.23 |
3.51 |
0.63 |
1.02 |
11.89 |
1.71 |
0.23 |
0.44 |
3.57 |
|
*LOI = loss on ignition |
|||||||||||
The composites were produced via a two-step stir casting technique as described by Alaneme et al [26, 27]. Prior to the stir casting process, charge calculation was done to determine the amount of groundnut shell ash (GSA) and silicon carbide (SiC) required for preparing 6 wt% and 10 wt% reinforcements in the proposed Al-Mg-Si based composite. The weight mix ratios of 0:1, 1:3, 1:1, 3:1 and 1:0 of GSA and SiC respectively were adopted for the production of varied grades of the 6 and 10 wt% reinforced composites.
Melting of the Al-Mg-Si alloy was carried out in a gas-fired crucible furnace at a temperature of about 750°C ± 30°C (above the liquid temperature of the alloy). The liquid alloy was then allowed to cool to a semi solid state at a temperature of about 600°C. Thereafter, reinforcement particulates of GSA and SiC that have initially been preheated at a temperature of 250°C were introduced into the melt. Also, 0.1 wt% magnesium (Mg) was also added for improved wettability. The composite slurry was stirred manually for 10 minutes before super heating to a temperature of 800°C ± 50°C for improved fluidity. A second stirring was performed at this super heat temperature using a mechanical stirrer operated at a speed of 400 rpm for 10 minutes; after which casting into prepared sand moulds inserted with chills was done. The composite grades produced with the corresponding sample designations are presented Table 3.
Table 3. Designation of Al-Mg-Si – GSA/SiC composite samples
|
6wt% |
|
10wt% |
||||
|
Samples |
Mix ratio GSA:SIC |
% GSA:SIC |
Samples |
Mix ratio GSA:SIC |
% GSA:SIC |
|
|
A1 |
0:1 |
0:100 |
B1 |
0:1 |
0:100 |
|
|
A2 |
1:3 |
25:75 |
B2 |
1:3 |
25:75 |
|
|
A3 |
1:1 |
50:50 |
B3 |
1:1 |
50:50 |
|
|
A4 |
3:1 |
75:25 |
B4 |
3:1 |
75:25 |
|
|
A5 |
1:0 |
100:0 |
B5 |
1:0 |
100:0 |
|
Corrosion behaviour
The corrosion behaviour of the composites was studied by weight loss method using mass loss and corrosion rate measurements. The corrosion test was carried out by gravimetric analysis of the test specimens in 0.3M H2SO4 and 3.5wt% NaCl solutions which were prepared following standard procedures [26, 27]. The specimens for the test were prepared by cutting the composites to size 15×15×10mm. The specimens were then mechanically polished with emery papers from 220 down to 600 grades to produce a smooth surface. The specimens were subjected to degreasing using acetone before rinsing in distilled water. Thereafter, the specimens were dried in air before they were immersed in static solutions of 3.5wt% NaCl and 0.3M H2SO4 at room temperature (25°C). The solution-to-specimen surface area ratio was about 150 ml/cm2, and the corrosion cells were exposed to atmospheric air for the entire period of the experiment. The gravimetric readings were taken at an interval of two days for a period of 44 days. The mass loss (mg/cm2) for each sample was evaluated in accordance with ASTM G31 standard recommended practice following the relation as described in [27]:
|
ml = CW/A |
(1) |
where ml is the mass loss (mg/cm2), CW is the cumulative weight loss (mg), and A is the total surface area of the sample (cm2).
The corrosion rate for each sample was evaluated from the weight loss measurements following the relation:
|
CR = KW/(ρAT) |
(2) |
where CR is corrosion rate (mmy), W is weight loss (g), D is the density (g/cm3), A is the area(cm2), T is time (hours), and K is a constant equal to 87500. The weight loss of the samples was determined using the Eq. 3:
|
W = Wi - Wf |
(3) |
where W is the weight loss (g), Wi is the initial weight (g) and Wf is the final weight (g).
Results and discussion
Corrosion behaviour in 3.5 wt% NaCl environment
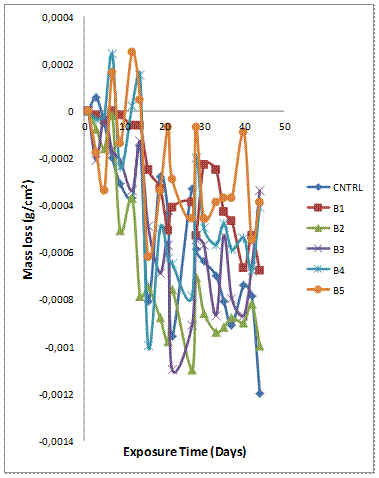
Figure 1(a-b) shows the variation of mass loss and corrosion rate against exposure time of the samples with 6wt% reinforcement immersed in 3.5% NaCl. It is observed from Figure 1(a) that the mass losses for all the samples (monolithic alloy, single reinforced composites and hybrid composites) were negative for most of the immersion time. This is an indication that the samples experienced weight gain and the films formed on the samples were stable enough to protect the samples from corroding in the environment.Sample A5 (single reinforced Al-Mg-Si– 6wt% GSA composite) and A1 (single reinforced Al-Mg-Si – 6wt% SiC composite) were observed to have superior corrosion to resistance respectively when compared with the hybrid composite compositions (A2, A3 and A4) and Al-Mg-Si Alloy (control sample). Similar observation has been reported in literature but for AMCs containing varied ratios of rice husk ash (RHA) and alumina as reinforcements [26]. Sample A2 (Al-Mg-Si - GSA/SiC 1:3 composites) is noted to be the least corrosion resistant of the hybrid composites produced. It is also noted that the corrosion behaviour of the composites is invariant to the weight ratio of GSA to SiC.
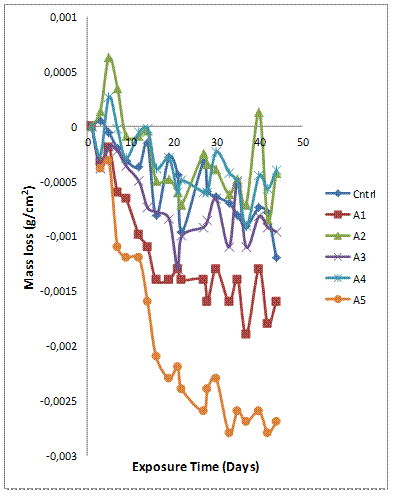
Figure 1(a). Variation of mass loss with exposure time for Al-Mg-Si/ 6wt% GSA-SiC composites in 3.5 % NaCl
The mass loss behaviour observed in Figure 1(a) is supported by the corrosion rate trends for the composite presented in Figure 1(b). The peak of the corrosion rate for most of the composites was observed on the fifth day of immersion with sample A2 (hybrid composite containing GSA and SiC in weight ratio 1:3) and A4 (hybrid composite containing GSA and SiC in weight ratio 3:1) exhibited the highest corrosion rates but was less than 0.04mmpy. An indication of the relatively superior corrosion rate of the single reinforced composites (A1 and A5) is observed on the five days of immersion where it is noted that both composites had averagely lower corrosion rates compared with the hybrid composite compositions. The corrosion rates of all the composites were observed to be relatively stable after fifth day of immersion in the 3.5% NaCl solution.
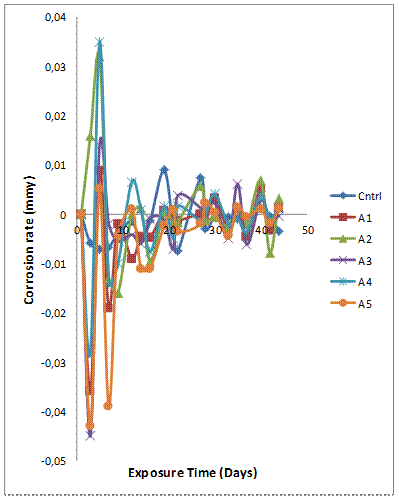
Figure 1(b). Variation of corrosion rate with exposure time for Al-Mg-Si/6wt% GSA-SiC composites in 3.5 % NaCl solution
Figure 2(a-b) shows the variation of mass loss and corrosion rate against exposure time of the samples with 10 wt% reinforcements immersed in 3.5% NaCl environment. It is observed from Figure 2(a) that all the samples (monolithic alloy, single reinforced composites and hybrid composites) experienced weight gain as reflected by the negative mass loss values. This suggests that the Al-Mg-Si alloy based composites containing 6 and 10 wt% GSA and SiC as reinforcements are corrosion resistant in NaCl environment and are hence suitable for use in marine environments. From Figure 2(a) it is observed that the hybrid composites (B2, B3 and B4) have superior corrosion resistance in comparison with the single reinforced composites (sample B1 and B5); unlike the mass loss trend observed for 6 wt% reinforced composites (Figure 1a). Similar observation has been reported for aluminium hybrid composites containing varied ratios of 10wt% bamboo leaf ash (BLA) and silicon carbide [27].
Figure 2(a). Variation of mass loss with exposure time for Al-Mg-Si/ 10wt% GSA-SiC Composites in 3.5 % NaCl solution
The superior corrosion resistance observed in the hybrid composites for 10wt % is attributed to the presence of silica in the composition of the GSA. Silica has been reported to impede the formation of Al4C3 phase formed as a result of interfacial reaction between the SiC and the Al (6063) alloy during the casting process. The formation of Al4C3 has been reported to promote the corrosion susceptibility of the composites [27].
The trend of the corrosion rate plot in Figure 2(b) shows that the peak corrosion rate was observed on the seventh day of immersion with sample B5 having the least corrosion resistance. However, the peak of the corrosion rate was less than 0.03mmpy for sample B5. The corrosion rate was stable from the 20th day to the last day of the experiment.It should be noted that the corrosion behaviour of single synthetic ceramic (Al2O3; SiC; SiO2) reinforced Al-Mg-Si based composites from earlier studies [28, 29] were reported to be suitable for use in marine environments due to stable and passive film formed by these materials when immersed in 3.5wt% NaCl solution (which is a representative composition of the saline content in a typical marine environment).
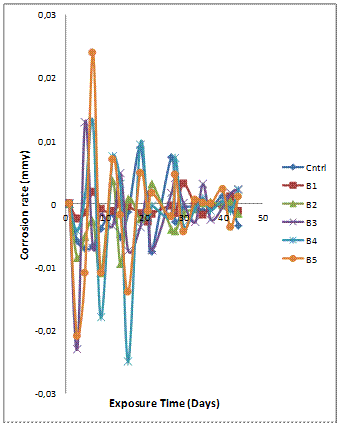
Figure 2(b). Variation of corrosion rate with exposure time for Al-Mg-Si/10wt% GSA-SiC composites in 3.5 % NaCl solution
The results from this research have shown that the incorporation of GSA as a complementing reinforcement to SiC did not have an adverse effect on the corrosion behaviour of the composites in NaCl environment.
Corrosion behaviour in 0.3 M H2SO4environment
Figure 3(a-b) shows the variation of mass loss and corrosion rate against exposure time of the samples with 6wt% GSA-SiC reinforcement immersed in 0.3M H2SO4 solution. It is observed that the mass loss for all samples increases with increasing exposure time (Figure 3a). This means that the passive film formed on the samples were unstable and could not provide adequate corrosion protection for the coupons. However, the composites with hybrid reinforcement compositions A2 and A4 (except sample A3) exhibited superior corrosion resistance in comparison with the single reinforced composites (sample B1 and B5).
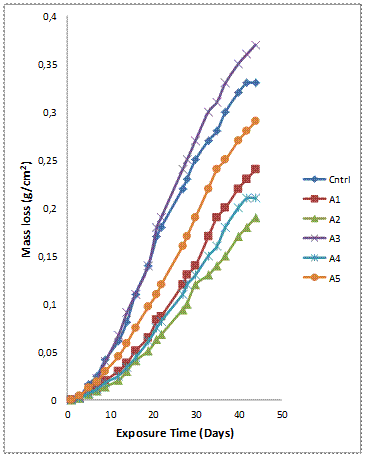
Figure 3(a). Variation of mass loss with exposure time for Al-Mg-Si/ 6wt% GSA-SiC composites in H2SO4 solution
The corrosion rate plot (Figure 3b) shows trends consistent with the observation in Figure 3(a) where the mass loss and corrosion rate values are highest for sample A3 (Al-Mg-Si - 3wt%GSA/3wt%SiC), and the sample A2 having the least mass loss and corrosion rate. Similarly the composites with intermediate mass loss values (Figure 3a) are observed to follow the same trend for the corrosion rate (Figure 3b).
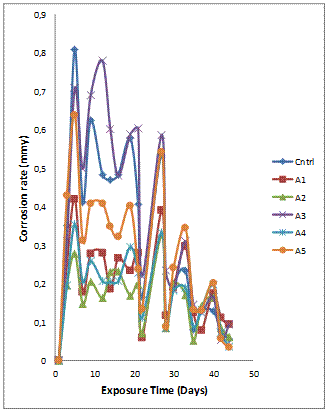
Figure 3(b). Variation of corrosion rate with exposure time for Al-Mg-Si/ 6wt% GSA-SiC composites in H2SO4 solution
Figure 4(a-b) shows the variation of mass loss and corrosion rate against exposure time of the samples with 10wt% reinforcement immersed in 0.3M H2SO4 solution. It is observed that the corrosion resistance of the single reinforced composites is superior to that of the hybrid composites contrary to the trend observed for the 6wt% reinforcement composite grades. Notwithstanding the seeming contradictory trends for both wt% reinforcements, the mass losses of the composites were not significantly affected by increase in the wt% of the reinforcing phase. Samples A4 and B4 with mix ratio of 75% GSA and 25% SiC have proven to have superior and consistent corrosion resistance among the hybrid counterparts at both 6 and 10wt% reinforcing levels (Figures 3a, 3b, 4a and 4b). The corrosion rate plot (Figure 4b) concurs with the observation in Figure 4(a). The least corrosion rate was observed in single reinforced Al-Mg-Si/10wt% GSA composites (sample B5).
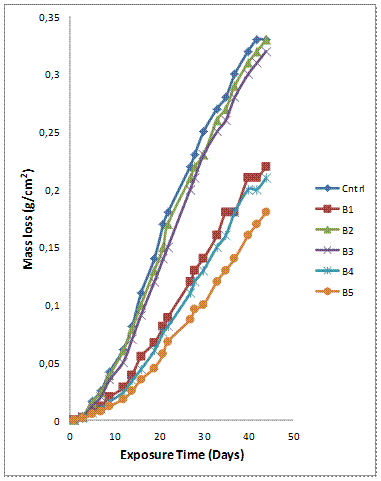
Figure 4(a). Variation of mass loss with exposure time for Al-Mg-Si/ 10wt% GSA-SiC Composites in 0.3M H2SO4 solution
The corrosion behaviour of silicon carbide reinforced Al-Mg-Si have been previously studied. Bobic [29] in his review established galvanic corrosion which occurs preferentially at the matrix-reinforcement interface as the corrosion mechanism of silicon carbide reinforced aluminium based composites. It is interesting to note that the addition of GSA to silicon carbide at 3 to 1 mix ratio (sample A4 and B4) resulted in enhanced corrosion resistance of the composites. The improved corrosion resistance exhibited by samples A4, B4 and B5 can be attributed to the combine action of silica and alumina in the GSA. Sample B4 and B5 have 75% and 100% GSA respectively as the reinforcement.
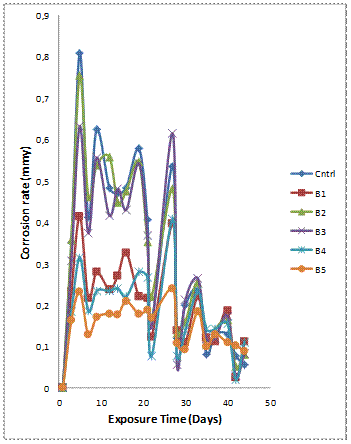
Figure 4(b). Variation of corrosion rate against exposure time for Al-Mg-Si/ 10wt% GSA-SiC Composites in 0.3M H2SO4 solution
The amount of silica present in the composites helps in inhibiting the formation Al4C3 phase which has an adverse effect on the corrosion resistance of the composites. Also, alumina present in the composites would reduce the possibility of galvanic corrosion between SiC and Al-Mg-Si in the hybrid composite. This is because resistivity of alumina (>14Ω) is far higher than that of SiC [10, 11], therefore, the tendency towards the formation of microgalvanic cells which brings about dissolution of Al-Mg-Si alloy preferentially to GSA-SiC is reduced.
Conclusion
The corrosion behaviour of Al-Mg-Si/GSA-SiC hybrid composites was investigated in 3.5% NaCl and 0.3M H2SO4 solutions. The following conclusions were drawn from the research:
1. The Al-Mg-Si alloy based composites containing 6 and 10 wt% GSA and SiC in varied weight ratios were resistant to corrosion in 3.5% NaCl solution.
2. The composites were more susceptible to corrosion in 0.3M H2SO4 solution (in comparison with the 3.5% NaCl solution).
3. It was noted that the Al-Mg-Si/6wt% GSA-SiC hybrid composite grades containing GSA and SiC in weight ratio 1:3 and 3:1 respectively exhibited superior corrosion resistance in the 0.3M H2SO4 solution compared to other composites produced for this series.
4. In the case of the Al-Mg-Si/10wt% GSA-SiC hybrid composite grades, the corrosion resistance was relatively superior for the composites containing a greater weight ratio of GSA (75% and 100%) in 0.3M H2SO4 solution.
References
1. Alaneme K. K., Bodunrin M. O., Mechanical behaviour of alumina reinforced AA (6063) metal matrix composites developed by two – step stir casting process, ActaTechnica Corviniensis, 2013, 3, p. 105-110.
2. Alaneme K. K., Aluko A. O., Production and age – hardening behaviour of borax pre-mixed SiC reinforced Al-Mg-Si alloy composites developed by double stir casting technique, The West Indian Journal of Engineering, 2012, 34(1-2), p. 80-85.
3. Mehandra K. V., Radhakrisna A., Characterization of stir cast Al-Cu (fly ash + SiC) hybrid metal matrix composites, Journal of Composite Materials, 2010, 44, p. 989-1005.
4. Bodunrin M. O., Alaneme K. K., Olusegun S. J., Influence of thermomechanical processing on the corrosion behaviour of aluminium (6063)-SiCp composites in NaCl and H2SO4environment,Science Journal Ubon Ratchathani University, 2011, 2(2), p. 17-25.
5. Escalera-Lozano R., Gutierrez C., Pech-Canul M. A., Pech-CAnul M. I., Degradation of Al/SiCp composites produced with rice-hull ash and aluminium cans, Journal of Waste Management, 2008, 1, p. 389-395.
6. Lancaster L., Lung M. H., Sujan D., Utilization of agro-industrial waste in metal matrix composites: towards sustainability, World Academy of Science, engineering and Technology, 2013, 73, p. 1136-.1144.
7. Tahamtan S., Halvee A., Emamy M., Zabihi M. S., Fabrication of Al/A206-Al2O3nano/micro composite by combining ball milling and stir casting technology, Materials and Design, 2013, 49, p. 347-359.
8. Naresh P., Development and characterization of metal matrix composite using red mud anindustrial waste for wear resistant applications, PhD Thesis, Department of MechanicalEngineering, National Institute of TechnologyRourkela, India, 2006, p. 23-34.
9. Alaneme K. K., Olubambi P. A., Corrosion and wear behaviour of rice husk ash – alumina reinforced Al-Mg-Si alloy hybrid composites, Journal of Materials Research and Technology, 2013, 2(2), p. 188-194.
10. Raju G. U., Gaitonde V. N., Kumarappa S., Experimental study on optimization of thermal properties of groundnut shell particulate reinforced polymer metal matrix composite, International Journal of Engineering and Science, 2012, 2(3), p. 433-454.
11. Prasad S. D., Krishna R. A., Production and mechanical properties of A356.2 /RHA composites, International Journal of Advanced Science and Technology, 2011, 33, p. 51-58.
12. Zhu J., Hihara L. H., Corrosion of continuous alumina-fibre reinforced Al-2 wt.% Cu-T6metal-matrix composite in 3.15 wt.% NaCl solution, Corrosion Science, 2010, 52, p. 406-415.
13. Arun Kumar M. B., Swany R. P., Evaluation of mechanical properties of A6061, fly ash and E-glass fiber reinforced hybrid metal matrix composites, ARPN Journal of Engineering and Applied Sciences, 2011, 6(4), p. 40-44.
14. Aigbodion V. S., Development of Al-Si-Fe/Rice husk ash particulate composite synthesis by double stir casting method, Usak University Journal of Material Science, 2012, 2, p. 187-197.
15. Atunaya C. U., Aigbodion V. S., Nwigbo S. C., Characterization of breadfruit seed hull ash for potential utilization in metal matrix composites for automotive application, Peoples Journal of Science and Technology, 2012, 2(1), p. 1-7.
16. Madakson P. B., Yawas D. S., Apasi A., Characterization of coconut shell ash for potential utilization in metal matrix composites for automoitveapplications, International Journal of Engineering Science and Technology (IJEST), 2012, 4, p. 1190-1198.
17. Naidu A. L., Sudarshan B., Krishna K. H., Study on mechanical behaviour of groundnut shell fiberre inforced polymer metal matrix composites, International Journal of Engineering Research and Technology, 2013, 2(2), p. 1-6.
18. Groundnut (Arachis hypogaea L.) (online) © International Crops Research Institute for the Semi-Arid Tropic. Available at: www.icrisat.org/crop-groundnut.htm.
19. Abdulazeez M. L., Jubril O. A., Ademola S. T., Economics of small scale agro-enterprises in Nigeria. A case study of groundnut processing among the rural women in Kwara State, Journal of Sustainable Development in Africa, 2012, 14(5), p. 54-64.
20. Adole M. A., Dzazu W. E., Umar A., Oraegbune O. M., Effect of groundnut husk ash blended cement on chemical resistance of concrete, Journal of Environmental Technology, 2011, 4(1), p. 23-33.
21. Folagbade O., George M., Groundnut shell ash stabilization of black cotton soil, EDJE, 2010, 15, p. 414-428.
22. Chawla N., Shen Y., Mechanical behaviour of particle reinforced metal matrix composites, Advanced Engineering Materials, 2001, 3(6), p. 357-370.
23. De-Salazaar J. M. G., Urena A., Manzabedo S., Barrena M. I., Corrosion behavior of AA6061 and AA7075 reinorced with Al2O3particles in aerated 3.5% chloride solution, potentiodynamic measurement and microstructure evaluation,Corrosion Science, 1999, 41, p. 529-545.
24. Pinto G. M., Jagannath N., Shetty A. N., The influence of silicon carbide reinforcement on pitting behaviour of aluminium, Corrosion Science, 2010, 38, p. 77-191.
25. Alaneme K. K., Corrosion behaviour of heat-treated Al-6063/SiCp composites immersed in 5wt% Nacl solution, Leonardo Journal of Science, 2011, 18, p. 55-64.
26. Alaneme K. K., Akintunde I. B., Olubambi P. A., Adewale T. M., Mechanical behavior of rice husk ash alumina hybrid reinforced aluminium based matrix composites, Journal of Materials Research and Technology, 2013, 2(1), p. 60-67.
27. Alaneme K. K., Ademilua B. O., Bodunrin M. O., Mechanical properties and corrosion behavior of aluminium hybrid composites reinforced with silicon carbide, and bamboo leaf ash, Tribology in Industry, 2013, 35(1), p. 25-35.
28. Alaneme K. K., Bodunrin M. O., Corrosion behavior of alumina reinforced Al(6o63) metal matrix composites, Journal of Minerals and Materials Characterization and Engineering, 2011, 10, p. 1153-1165.
29. Bobic B., Mitrovic S., Bobic M., Bobic I., Corrosion of metal matrix composites with aluminium alloy substrate, Tribology in Industry, 2010, 32, p. 3-11.