Effect of carburizing temperature and time on mechanical properties of AISI/SAE 1020 steel using carbonized palm kernel shell
Olanike Mary OLUWAFEMI1, Samuel Ranti OKE2, Iyiola Olatunji OTUNNIYI2, and Fatai Olufemi ARAMIDE2*
1Engineering Materials Development Institute (E.M.D.I.), Akure, Ondo State, Nigeria
2Metallurgical and Materials Engineering Dept., Federal University of Technology PMB 704, Akure, Ondo State, Nigeria
E-mails: marypaul_nike@yahoo.com, sroke@futa.edu.ng iootunniyi@futa.edu.ng *foaramide@futa.edu.ng
*Corresponding author, phone: +2348038509288
Abstract
The effects of varied carburizing temperatures and holding time on the mechanical properties of AISI/SAE1020 steel have been investigated. Standard test samples prepared from the steel sample were subjected to pack hardening process using carbonized palm kernel shell as a carburizer at 800°, 850°, 900° and 950°C and held for 60, 90 and 120 minutes, quenched in oil and temper at 500°C for 60 minutes. After pack hardening process, the test samples were subjected to tensile, impact and hardness tests. and from the data obtained Ultimate tensile strength (UTS) and impact strength were calculated, the case and the core hardness of the carburized samples were taken and optical microscope was also used to observe the micro structural features of the case hardened, quenched and tempered samples. It was observed that at 800°, 850° and 900°C, the UTS and the micro hardness initially reduces to minimum and then increased as the carburizing temperature increased, but at 950°C, it was observed that the UTS increases with increase in holding time. It was concluded that the optimum combination of mechanical properties is achieved at the carburizing temperature of 950°C soaked for 120 minutes followed by oil quenching and tempered at 500°C for 60 minutes.
Keywords
Carburizing temperature; Carbonized palm kernel shell; Holding time; Ultimate tensile strength
Introduction
Low carbon steel often does not meet the requirements for manufacturing certain end products due to their low carbon content. To meet requirements for high carbon steel, case hardening is usually done on the low carbon steels. Case hardening is useful for parts that need to be hard externally to endure wear and tear, but soft internally to withstand shock [1-2]. The mild steel can be subjected to heating at a particular temperature, until it is bright red, and then immersed into a carbon-rich media, so that carbon atoms penetrates the outer surface and bring about a surface structure of higher hardness [3-4]. The media can be solid, liquid or gas. When in solid form, is it called pack carburizing, the mild steel is packed in a well-sealed box in the presence of any carbon containing material or carburizer and then heated to a temperature within the austenitic region of the steel which generally is between 850°C and 950°C for mild steels and then held at that temperature for a period of time [5]. At this austenitic region; there is high solubility for carbons and the case depth of carburized steel is a function of carburizing time and the available carbon potential at the surface [6]. It shows that the longer it is held, the deeper the carbon penetration into the iron or steel. Many workers have investigated different materials such as animal bone, sea shell and palm kernel as carburizer in pack hardening of steel [7], activated carbon as carburizer [8], cassava leaf as carburizer [9], graphite, charcoal and palm kernel as carburizer [2], egg shell as carburizer [10]. All the work reported that the carburizers had a great influence on the mechanical properties of mild steel. After hardening due to carburization, tempering operation can be carried out to temper the steel to reduce the brittleness created by the hardening process. The steel may be heated in a furnace or a bath of oil, sodium and potassium nitrate mixture or lead. The temperature to which the steel is heated during tempering determines the final hardness of the tempered steel.
Whichever method used to harden the case, naturally, the process must be controlled and the results verified according to specifications. Depending on the application, it is important to test the resulting material to determine the effectiveness of the treatment. Properties such as ultimate tensile strength, surface hardness, impact test, micro hardness must be determined and the resulting micro structural characterized.
The last author together with other co-authors [1, 8] had earlier reported on the effects of carburization temperature and soaking time on the mechanical properties of mild steel using activated carbon and pulverized bone as carburizers. But the time used during the tests range between 15 minutes to 45 minutes.
The aim of this work is to produce case hardened SAE1020 steel of optimum mechanical property. This is intended by investigating the effects of longer soaking time at the carburization temperatures on the investigated mechanical properties.
Material and method
AISI/SAE 1020 mild steel used in this research work with the chemical composition shown in Table 1 was sourced from Universal steel company, Ogba Industrial Estate Ikeja, Lagos. Palm kernel shell was also sourced from Oja-Oba market in Akure; it was dried, grinded and pulverized to powder, while industrial engine oil (ABRO) was used as the quenching medium.
Table 1. Chemical composition of the mild steel sample
|
Element |
C |
Si |
S |
P |
Mn |
Ni |
Cr |
Mo |
V |
Cu |
|
Avg. content |
0.1999 |
0.1548 |
0.0594 |
0.0459 |
0.5826 |
0.131 |
0.1105 |
0.016 |
0.0013 |
0.384 |
|
Element |
W |
As |
Sn |
Co |
Al |
Pb |
Ca |
Zn |
Fe |
- |
|
Avg. content |
0.0076 |
0.0051 |
0.0377 |
0.0098 |
0.0028 |
0 |
0.0001 |
0.0048 |
98.247 |
- |
Pack carburizing operation
The mild steel (AISI/SAE 1020) was machined to standard impact and tensile test sample configuration. The prepared test samples were embedded in a steel pot containing pulverized carbonized palm kernel shell. The steel pot was tightly sealed with clay cover to prevent the escape of carbon and unwanted furnace gas from entering the steel pot during heating. The furnace temperature was adjusted to the required temperature (800°C, 850°C, 900°C and 950°C for each stage respectively) and the loaded steel pot was charged into the furnace. When the furnace temperature reaches the required carburizing temperature, it was then held/soaked at the temperature for the required time (60, 90 and 120 minutes). After the material was held at the specified time, the steel pot was removed from the furnace and the material was quenched in industrial engine oil. The carburized test samples were then tempered at a temperature of 500°C, held for one hour, and then cooled in air. After the cycles of heat-treatment, the test samples were subjected to tensile test, impact test and hardness test.
Mechanical testing
Tensile tests were carried out at room temperature on the carburized samples following specifications described in American standard testing and measurement method ASTM E36 [11] using the Instron universal tensile testing machine operated at a strain rate of 10-3/s. Ultimate tensile strength was evaluated from the tension test. To ensure reliability of the values generated, three repeat tests were performed for each grade of the carburized samples.
The impact tests are performed after the ‘V-notch method using the Hounsfield balance testing machine’ with ASTM D256 specification [12]. Prior to mounting the machine, the test sample is notched to a depth of 2mm with v-shaped hand file. The notched test sample was then mounted on the impact-testing machine, which is operated to apply a constant impact force on the test sample.
A hardness test was conducted using LM700 Spec Leco micro-hardness tester. The hardness test values were obtained by forcing a hardened steel ball indenter of 10mm diameter into the surface and core of each grade of polished carburized mold steel samples under a static load. Several hardness tests were performed on each sample and the average of values taken within the range of ± 2% was recorded as the hardness of the specimen.
Micro structural examination
Nikon Eclipse ME-600 Metallurgical Microscope with accessories for image analysis was used for optical microscopic investigation of the carburized mild steel samples. The samples to be examined were metallographically polished and etched before microscopic examination was performed. The polished samples were etched in 2% Nital solution before they were viewed under the microscope.
Results and discussion
The results obtained are recorded in Table 2 and Figures 1 to 6, while the micrographs of the samples as obtained from optical metallurgical microscope are shown in Figures 7 to 11.
Table 2. Details of the mechanical test conducted on the carburized, tempered steel
|
Treatment (°C) |
Holding time (min) |
Micro-hardness of case (HV) |
Micro-hardness of core (HV) |
UTS (N/mm2) |
Impact (J) |
Strain (%) |
Modulus (N/mm2) |
|
800 |
60 |
265.8 |
337.5 |
824.37 |
47.19 |
0.064 |
12880.78 |
|
90 |
240.4 |
224.7 |
723.93 |
58.48 |
0.143 |
4049.93 |
|
|
120 |
257.4 |
250.5 |
740.76 |
34.54 |
0.074 |
10010.27 |
|
|
850 |
60 |
236.9 |
290.15 |
740.38 |
41.34 |
0.068 |
10207.35 |
|
90 |
194.2 |
229.2 |
720.98 |
43.66 |
0.069 |
4049.93 |
|
|
120 |
199.85 |
236.0 |
1010.81 |
42.98 |
0.143 |
5381.96 |
|
|
900 |
60 |
340.8 |
229.3 |
850.98 |
26.38 |
0.121 |
5860.83 |
|
90 |
207.6 |
205.9 |
555.50 |
15.64 |
0.13 |
3636.69 |
|
|
120 |
287.5 |
288.8 |
856.34 |
26.66 |
0.012 |
71361.67 |
|
|
950 |
60 |
263.3 |
274.25 |
730.74 |
19.99 |
0.038 |
19230.06 |
|
90 |
291.2 |
243.25 |
961.76 |
13.60 |
0.00382 |
251769.6 |
|
|
120 |
357.95 |
347.9 |
970.99 |
15.50 |
0.034 |
24436.47 |
Mechanical properties of the carburized steel
The result of the tensile properties of the carburized steel with respect to increasing carburizing temperature and soaking time is presented in Figures 1. From the figure it is observed that for steel samples carburized at 800°C, 850°C and 900°C, there is a general decrease in the ultimate tensile strength with increase in holding time (from 60 to 90 minutes). An increase in the UTS was again observed when the soaking time was further increased to 120 minutes. For the samples carburized at 800°C, the ultimate tensile strength (UTS) of the sample soaked for 60minutes was 824.37N/mm2, a reduction in UTS value (723.93N/mm2) was noticed as the soaking time increases to 90minutes, while at 120 minutes; the value slightly increases again to 740.76N/mm2. The same trend was observed for samples carburized at 850°C, and 900°C. At carburization temperature 850°C, the highest UTS of value 1010.81N/mm2 were gotten from the sample soaked for 120 minutes. But at 900°C, the UTS reduce to the minimum value (555.5 N/mm2) for the samples soaked for 90minute which later to 856.34 N/mm2 when soaked for 120minutes. As the carburizing temperature was increased to 950°C, the UTS value increases with increase in the soaking time (from 60 to 120 minutes).
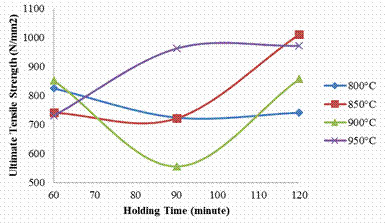
Figure 1. Effects of carburizing temperature and holding time on the ultimate tensile strength of the sample
Generally, steel samples soaked for 120 minutes tend to possess improved strength when compared with samples soaked for 60 and 90 minutes.
Figure 2 shows the variation in strain of the carburized steel samples with respect to carburizing temperature and holding time. It was observed that at 800°C, the minimum strain value was 0.064 when soaked for 60 minutes and later increased to 0.143 when soaked for 90 minutes, also, a drastic reduction in value (0.0074) was observed when soaked for 120 minutes. Subsequently, at 850°C the stain values of the samples increases with increase in soaking time (i.e. 0.068, 0.069 and 0.143 when soaked for 60, 90 and 120 minutes). An initial rise in strain values (0.121, 0.13) was also observed at 900°C when soaked for 60 and 90 minutes, but it later reduced to 0.012 at the soaking time of 120 minutes.
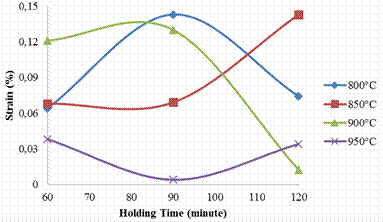
Figure 2. Effects of carburizing temperature and soaking time on the engineering strain of the carburized samples
This lost in ductility can be attributed to the volume fraction of carbide formed in the material [12]. With further increase in the carburizing temperature to 950°C for the sample soaked for 60 minutes, the strain value was 0.038, followed by a drastic reduction to 0.00382 when soaked for 90 minutes and later increased to 0.034 when soaked for 120 minutes.
The Young’s modulus of elasticity of the samples is presented in Figure 3. The sample carburized at 800°C and soaked for 60minutes gives the maximum value of 12880.78 N/mm2 and later reduced to 4049.93N/mm2 when soaked for 90 minutes, while the sample held for 120 minutes was observed to rise again to 10010.27N/mm2. This shows a sinusoidal curve as the holding increases. At 850°C, initially the value was very high (10207.35N/mm2) when soaked for 60 minutes, but it later reduces drastically (4049.93N/mm2) at the soaking time of 90 minutes before it picks up and increased in value when soaked for 120 minutes. The same trend was maintained as the temperature was increased to 900°C. Because of the stiffness of the carburized steel a sudden sharp rise was observed in the sample carburized at 950°C thus attaining the peak value of 251769N/mm2 at 90 minutes then followed by another sharp drop.
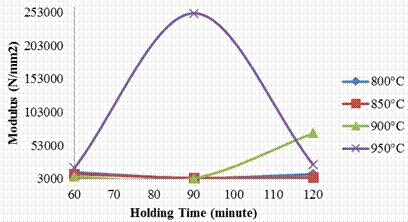
Figure 3. Effects of carburizing temperature and holding time on the young’s modulus of elasticity of the sample
This shows that the sample exhibits the highest stiffness as the holding time increases due to more carbon diffusion into the samples. This can be explained through the study of Iron-Iron Carbide phase diagram for the steel specification as reported by Massalki, (1990) [13]. The carburizing temperature of 950°C is well within the austenitic region for the specification of steel, which means that the solid solution of iron with carbon is favored at this temperature.
Figure 4 illustrates the variation of impact strength with respect to the holding time of tempered carburized steel. Swing-like curves were observed during examination of the plots. In the samples carburized at 800 and 850°C, an increase in the impact values was observed with the earlier attaining an impressing peak value of 58J at 90 minutes holding time. The later shows that the impact energy absorbed by the sample will keep increasing as the holding time increase as explained by the linear trend in the plot.
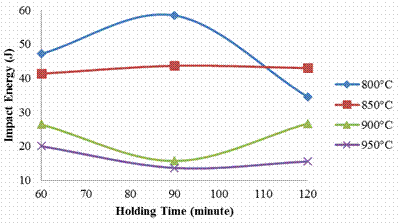
Figure 4. Effects of carburizing temperature and holding time on the impact strength of the sample
A further increase in the carburizing temperature results in declination in the impact energy value, these is depicted by the plots of the samples carburized at 900 and 950°C as the steel tends to sacrifice its impact energy for some other mechanical properties.
Figures 5 and 6 are the graphical representations showing the variation of micro hardness of the carburized steel.
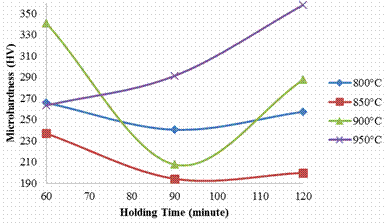
Figure 5. Effect of carburizing temperature and holding time on case micro hardness of the sample
It was observed that all the samples carburized at 800, 850 and 900°C follow the same trend beginning from high hardness values at the case and core when soaked for 60 minutes (265.8HV for case and 337.5HV for core) and gradually decreases at 90 minutes holding time, (240.4HV and 224.7HV for core) but gradually begin to increase again as the holding time is further increased to 120 minutes (257.4HV for case and250.5HV for core); thus attaining the respective peak hardness values at 120 minutes holding time.
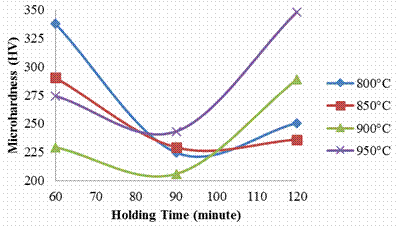
Figure 6. Effect of carburizing temperature and holding time on core micro hardness of the sample
For the sample carburized at 950°C, it was observed that the case hardness increases with increase in time, (i.e. 263.3HV, 291.2HV and 357.95HV for the soaking temperatures 60, 90, and 120 minutes) while the core hardness rose initially to hardness value of 274.25HV when soaked for 60 minutes and later reduces to 243.25HV when soaked for 90 minutes, as the soaking time was increased to 120 minutes, it exhibits the highest hardness value of 347.9HV. The increase reported in the samples at 120 minutes has been attributed to the diffusion of carbon in the sample [14]. When comparing with the hardness of as received sample, it is glaring that there was carbon enrichment in the carburized sample, thus, the optimum carburization process route that shows the trend of hard case and softer core was gotten from the sample carburized at 950°C and soaked for 90 and 120 minutes
Effects on microstructure of the carburized steel
Figure 7 show (a) the case or surface micrograph of the mild steel and (b) shows the micrograph of the core of the selected mild steel. No difference is observed as no treatment has been given to it and thus it serves as the control sample for this experiment. As conventionally established, this untreated steel microstructures show a pearlite structure in a matrix of ferrite.
(a) (b)
(b)
Figure 7. Microstructures of as-received mild steel showing (a) the case and (b) the core, 200x
Sample treated at 800°C and tempered at 500°C
Figure 8 shows the photomicrographs of carburized steels treated at 800°C and tempered at 500°C. Figure 8(a) indicates the structure of the surface/case when held for 60 minutes in the furnace while 8(b) is the structure of the core under the same condition. While tempered martensite was observed in the core along some sparsely distributed ferrite, the case contain some dark spots identified to be some carbon deposits which thus enhance some of its mechanical properties as earlier discussed.
It is observed in Figure 8(c) that the diffusion of carbon is more intense when it was held for 90 minutes thus leaving more of ferrite matrix at the skin of the steel. This finding is found to be true on critical observation of the core in Figure 8(d) that contain more of pearlite and tempered martensite with ferrites forming along the grain boundaries. Figure 8(e) and (f) show the micrographs of both the case and core respectively of the carburized steel when held for 120 minutes. This corresponds to some findings in literature review [15-16]. This holding time allows thorough diffusion of carbon forming more of black spots in the core as compared to the case. These spots, which is identified as the carbon deposits is found to be in the matrix of tempered martensite.
(a) (b)
(b)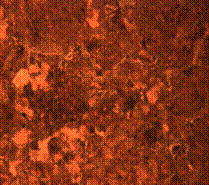
(c)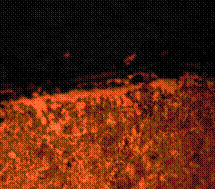 (d)
(d)
(e)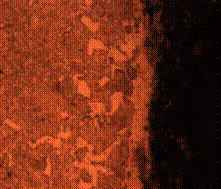 (f)
(f)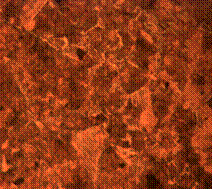
Figure 8. Microstructures of carburized tempered mild steel treated at 800°C and tempered at 500°C showing (a) the case of the specimen held for 60 minutes; (b) the core of the specimen held for 60 minutes; (c) the case of the specimen held for 90 minutes;(d) the core of the specimen held for90 minutes; (e) the case of the specimen held for 120 minutes and (f) the core of the specimen held for 120 minutes
Sample treated at 850°C and tempered at 500°C
Figure 9 shows the micrographs of the steel treated at 850°C, tempered at 500°C.
(a)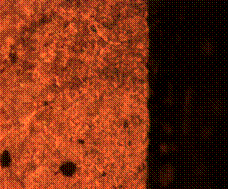 (b)
(b)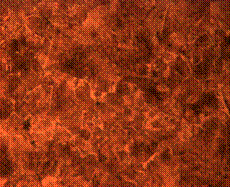
(c)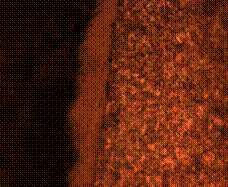 (d)
(d)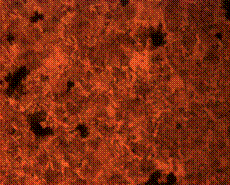
(e)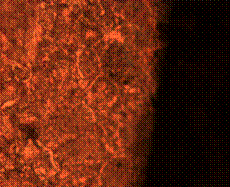 (f)
(f)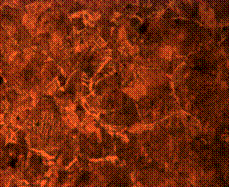
Figure 9. Microstructures of carburized tempered mild steel treated at 850°C and tempered at 500°C showing (a) the case of the specimen held for 60 minutes; (b) the core of the specimen held for 60 minutes; (c) the case of the specimen held for 90 minutes;(d) the core of the specimen held for90 minutes; (e) the case of the specimen held for 120 minutes and (f) the core of the specimen held for 120 minutes
There is early formation of tempered martensite when held for 60 minutes especially at the core as shown in Figure 9(b); the dark spots observed in Figure 9(a) are inclusion possibly in the course of rolling. A finer structure is observed in Figure 9(c) when the steel is held for 90 minutes with tempered martensite formed at the core. The dark spots observed at the core (Figure 9(d)) could be excess carbon contained in the cementite forming the tempered martensite. Complete tempered martensite is formed both at the surface and in the core when the steel is held for 120 minutes (Figure 9(e) and (f)). This thus account for the improvement in some of the mechanical properties as have been discussed earlier.
Sample treated at 900°C and tempered at 500°C
Figure 10 (a and b) are the micrographs of both the case and the core of the sample when held for 60 minutes in the furnace during tempering. It is observed to consist of higher degree of tempered martensite at the core with fine ferrite matrix at the case environs.
(a)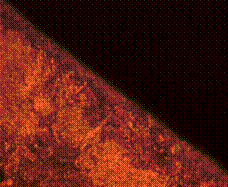 (b)
(b)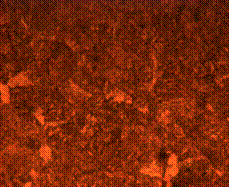
(c) (d)
(d)
(e)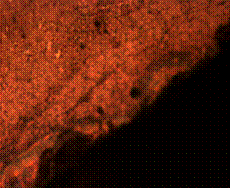 (f)
(f)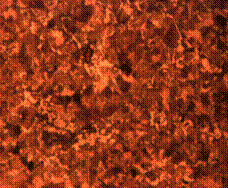
Figure 10. Microstructures of carburized tempered mild steel treated at 900°C and tempered at 500°C showing (a) the case of the specimen held for 60 minutes; (b) the core of the specimen held for 60 minutes; (c) the case of the specimen held for 90 minutes;(d) the core of the specimen held for90 minutes; (e) the case of the specimen held for 120 minutes and (f) the core of the specimen held for 120 minutes
When it is held for 90 minutes, the structure observed is no different from the control sample exhibiting mainly pearlite and ferrite only (Figure 10 (c and d)). Tempered martensite, pearlite, ferrite matrix along with some carbon deposit resulting from the carburizer was observed in the core of the sample held for 120 minutes (Figure 10 (f)). The case of the same sample however, shows fine structures of tempered martensite and carbon splashes with some inclusions at the skin environs (Figure 10 (e)).
Sample treated at 950°C and tempered at 500°C
Figure 11 shows the micrographs of the steel treated at 950°C, tempered at 500°C.
(a)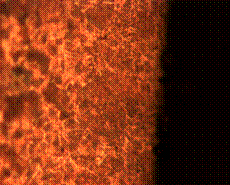 (b)
(b)
(c)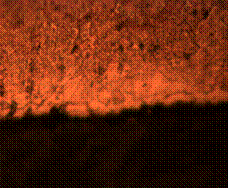 (d)
(d)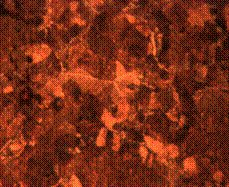
(e)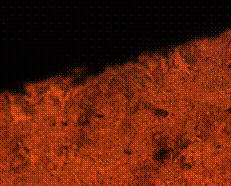 (f)
(f)
Figure 11. Microstructures of carburized tempered mild steel treated at 950°C and tempered at 500°C showing (a) the case of the specimen held for 60 minutes; (b) the core of the specimen held for 60 minutes; (c) the case of the specimen held for 90 minutes;(d) the core of the specimen held for90 minutes; (e) the case of the specimen held for 120 minutes and (f) the core of the specimen held for 120 minutes
In Figure 11(a) it was observed that the diffusion of carbon from the carburizer is just beginning as shown at the skin structure exhibiting finer structure in comparison to the core (see Figure 11(b)) of the same sample which was observed to possess a coarse and dense pearlitic structure. Figure 11(c) and (d) is microstructure of the sample held for 90 minutes; in it fully tempered martensite structure is displayed at the core in Figure 11(d) and fine structure of major ferrite at the skin region thus resulting into enhanced mechanical properties. When the sample is held for optimum time of 120 minutes, the structure both at the skin and the core is observed to be fully tempered martensite (Figure 11(e and f)).
Conclusions
From the discussion so far it is concluded that:
1. The mechanical properties of mild steels were found to be strongly influenced by the process of carburization, carburizing temperature and holding time using carbonized palm kernel shell;
2. Some conversion of carbon-enriched skin austenite to martensite can be inferred from the microstructure and the increase in hardness in most of the samples;
3. Optimum combination of mechanical properties is achieved at the carburizing temperature of 950°C soaked for 120 minutes followed by oil quenching and tempering at 500°C for 60 minutes;
4. The responses were mostly sinusoidal over time and detailed explanation and investigation into this response will be good for further studies.
References
1. Aramide F. O., Ibitoye S. A., Oladele I. O. and Borode J. O., Pack carburization of mild steel, using pulverized bone as carburize optimizing process Parameters, Leonardo Electronic Journal of Practices and Technologies, 16, 2010, p. 1 -12.
2. Alagbe M., Effects of some carburizing media on surface hardening of low carbon steel. Journal of Sciences and Multidisciplinary Research, 2011, 3, p. 1-7.
3. Ihom A. P., Nyior G. B., Ambayin M., Surface Hardness improvement of mild carbon steel using arecaceae waste flower droppings, The Pacific Journal of Science and Technology, 2012, 13(1), p. 133 -138.
4. Okongwu D. A., Assessment of the efficacy of some carbonate minerals as energizers in pack carburization of mild steel, J. NSE, 1989, p. 30-35.
5. Ihom A. P., Nyior G. B., Suleiman M. U., Ibrahim G. Z. Improving surface hardness of steel using rice husk waste, Nigerian Journal of Tropical Engineering, 2011, 1(2), p. 107-115.
6. Ihom A. P., Nyior G. B., Alabi O. O., Segun S., Nor I. J., Ogbodo J. N., The potentials of waste organic materials for surface hardness improvement of mild steel, International Journal of Scientific and Engineering Research, 2012, 3(11), p. 1-20.
7. Oyetunji A., Adeosun S. O., Effect of Carburizing process Variables of Mechanical and Chemical properties of Carburized Mild Steel, Journal of Basic and Applied Sciences, 2012, 8, p. 319-324.
8. Aramide F. O., Ibitoye S .A., Oladele I. O., Borode J. O., Effect of Carburization Time and Temperature on the Mechanical properties of Carburized Mild steel, using Activated Carbon as Carburizer, Journal of Materials Research, 2009, 12(4), p. 483-487.
9. Ibironke O. J., Falaiye A., Ojumu T. V., Odo E. A., Adewoye O. O., Case-depth studies of pack cyaniding of mild steel using cassava leaves, Materials and Manufacturing Processes, 2004, 19(5), p. 899-905.
10. Ihom A. P., Nyior G. B., Nor I. J., Ogbodo N. J., Investigation of egg shell waste as an enhancer in the carburization of mild steel, American Journals of Materials Science and Engineering, 2013, 1(2), p. 29-33.
11. American Society of Testing and Materials, Standard test method for tensile property of metallic materials, ASTM 2014, ASTM A370-E8M.
12. American Society of Testing and Materials, Standard test method for impact testing of metallic materials, ASTM 2014, ASTM A370-E23.
13. Massalki T. B. (Ed.), Binary alloy phase diagrams, 2nd ed., 1990, Vol. 1, ASM International.
14. Okongwu D. A., Assessment of the efficacy of some carbonate minerals as energizers in pack carburization of mild steel, J. NSE, 1989, p. 30-35.
15. Shrager A. M., Elementary metallurgy and metallography, 2nd Ed., Dover Publications New York, 1961, p. 175-176.
16. Higgins R. A., Properties of engineering materials 6th edition, Published by Hodder and Stoughton Educational, Great Britain, 1983, p. 199-200.