Chlorococcalean microalgae Ankistrodesmus convolutes biodiesel characterization with Fourier transform-infrared spectroscopy and gas chromatography mass spectroscopy techniques
Swati SONAWANE1*, Sanjaykumar DALVI2, and Raghunath POKHARKAR1
1Department of Chemistry, S. N. Arts, D. J. M. Commerce & B. N. S. Science College, Sangamner, Dist Ahmednagar, (M.S.) 422605, India
2Savitribai Phule Pune University, Ganeshkhind, Pune, (M.S.), 411007, India
E-mails: swatis2003@yahoo.co.in; snd_rang@yahoo.co.in ; raghunathpokharkar@rediffmail.com
* Corresponding author, phone: +91- 9326710125
Abstract
The Chlorococcalean microalgae Ankistrodesmus convolutes was found in fresh water Godawari reservoir, Ahmednagar district of Maharashtra State, India. Microalgae are modern biomass for the production of liquid biofuel due to its high solar cultivation efficiency. The collection, harvesting and drying processes were play vital role in converting algal biomass into energy liquid fuel. The oil extraction was the important step for the biodiesel synthesis. The fatty acid methyl ester (FAME) synthesis was carried through base catalyzed transesterification method. The product was analyzed by using the hyphened techniques like Fourier Transform-Infrared spectroscopy (FT-IR) and Gas Chromatography Mass Spectroscopy (GCMS). FT-IR Spectroscopy was results the ester as functional group of obtained product while the Gas Chromatography Mass Spectroscopy was results the six type of fatty acid methyl ester with different concentration. Ankistrodesmus convolutes biodiesel consist of 46.5% saturated and 49.14% unsaturated FAME.
Keywords
Biodiesel; Transesterification; Ankistrodesmus convolutes; Fourier Transform-Infrared spectroscopy; Gas chromatography; Mass spectroscopy
Introduction
An evaluating the potential renewable energy sources as alternative to fossil fuel is the vital challenge for each developing country. The demand of liquid fuel energy is increases continuously due to rapid industrialization and population. The basic non-renewable energy sources are petroleum, coal, natural gas, hydro and nuclear [1]. The 45% of hydrocarbon liquid fuel energy was obtained from fossil fuel based material which was the key economic driver in India [2] but the fossil fuel was exhausted with alarming rates [3]. The emission of air pollutants like NOx, SOx, CO, particulate matter and volatile organic compound after combustion fossil fuel atmospheric pollution and contribute for the green house gas emission (GHG) [4]. The high prices of hydrocarbon energy fuel with the dwindle feedstock of energy move towards the renewable energy sources. Recently, the research work focused on the modern clean energy feedstock like algae [5].
The aquatic alga biomass was represents the very interesting source of energy due to its higher photosynthetic activity as compare to terrestrial plant [6]. The diverse group of algae were comprises as macro and micro alga. It can be produce 1000-4000 gallon/ acre/yr oil and cultivated in large open pond or in closed photo bioreactors located on non-arable land. The microalgae produced high concentration of valuable compounds such as lipid, protein and pigment [7-9]. The growth of microalgae and lipid productivity is depending on growth medium composition, physical parameters and type of metabolism [10]. The rate of water consumption during the cultivation of microalgae is low and it possessing short harvesting cycle than the conventional crop cycle, hence it is better advantage to carry out the algal biomass as biofuel [11-13].
The aim of this research work is to study the isolation and identification of microalgae species from fresh water reservoir, medium selection and culture cultivation, algae growth study, base catalyzed transesterification reaction for FAME synthesis, FT-IR Spectroscopy and Gas Chromatography Mass Spectroscopy analysis of components of biodiesel product.
Material and method
The microalgae were collected by plankton net (20 µm pore size) from fresh water Godawari reservoir, Ahmednagar (Maharashtra), India. The microalgae sample was collected in clean plastic container from sampling location. The sample was immediately brought for algal studies. The water sample was observed on the spot in natural condition. The morphology of pure strains was regularly examined under an optical microscope and identified with the help of standard literature (Figure 1).
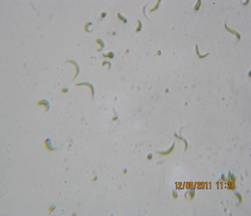
Figure 1. Ankistrodesmus convolutes microalgae
The Bold basal media used for culturing of Ankistrodesmus convolutes (Figure 1). The content of the media was NaNO3 (0.75), K2HPO4. 3 H2O (0.075), K H2PO4 (0.175), CaCl2 .H2O (0.025), MgSO4.7H2O (0.075), NaCl (0.025), EDTA (0.0000045), FeCl3.6 H2O (0.000582), MnCl2.4 H2O (0.000246), ZnCl2.6 H2O (0.00003), CoCl2.6 H2O (0.000012), Na2Mo2 H2O (0.000024). 10 ml of water samples were transferred to a 500 ml conical flask containing 200 ml of sterilized BBM and then incubated on a rotary shaker at 27°C and 150 rpm under continuous illumination using white fluorescent light at intensities of 40 µmol /m2/ s. Every two days, the flasks were examined for algal growth using an optical microscope (Figure 1), with serial dilutions being made in BBM from flasks showing growth.
Subcultures were made by inoculating 50 µL culture solution onto Petri plates containing BBM solidified with 1.5% (w/v) of bacteriological agar. These procedures were repeated for each of the original flasks. The Petri plates were incubated at 27°C under continuous illumination for two weeks. The purity of the culture was confirmed by repeated plating and by regular observation under a microscope. The growth curves of the Ankistrodesmus convolute strain in BBM media was study by determining the optical density of samples at 680 nm using a UV-Spectrophotometer. The microalgae cells were harvested in the stationary phase by centrifugation at 5000 rpm for 5 min and the cells were washed twice using distilled water. The cell pellets were dried at 60˚C for 2 days and placed in desiccators until constant weight. Dry weight of cells was obtained using an analytical balance. The lipid content of microalgae was extracted by using the Bligh and Dyer method [14]. After cell drying, algal powder was mix with chloroform–methanol (1:2) solvent for 30 min. Algal solid was removed by centrifugation at 5000 rpm for 5 min. The residual solid and lipid separated by solvent extraction procedure.
The Chemical conversion of algal oil to fatty acid methyl ester by alkali catalyzed transesterification method which involves multiple steps of reaction between triglyceride or fatty acid and alcohol. The methanol was used frequently for the commercial development due to its low cost and its physical and chemical advantages [15] [16]. The base catalyzed reaction was performed by using potassium methoxide with excess of methanol [17] [18]. The reaction is carried in a round bottom flask. The microalgae oil mixed with methanol then potassium hydroxide in methanol added. The reaction mixture was continuously stirring. The reaction carried out at 60°C for 60 minutes. At room temperature, biodiesel product phase was wash with distilled water to remove glycerol content and water soluble impurities by giving 2-3 time water wash to product with heating 85°C. It was preserved in airtight container and used for further analysis (Figure 2). The fatty acid methyl esters were analyzed by standard method FT-IR Spectroscopy and GCMS.
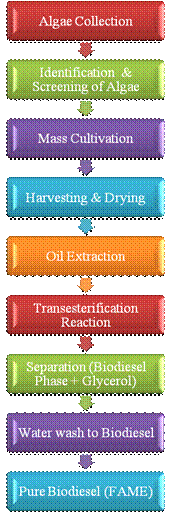
Figure 2. Process for Biodiesel production from microalgae
Results and discussion
The isolated microalgae strain belonging from Green algae, the division of Chlorophyta, class of Chlorophycae, family of Chlorococcaceae, order of Chlorococcales, genus Ankistrodesmus and species convolutes. Under the optical microscope Ankistrodesmus convolutes (Figure 1) was observed. It is unicellular spirally twisted around one another in the mid region, but free at the ends, 22.54-30.16µm long 2.17-3.20 µm broad.
The growth cycle of Ankistrodesmus convolutes in BBM is shown in Figure 3. Cells in BBM medium 1day lag period and reached the exponential phase within 3-8 days. By approximately12-14 days cells reached the stationary phase, after 15 days optical density increases. Lipid productivity and biomass productivity measured gl-1d-1 shown in Figure 4. The dry biomass of microalgae was found 0.047gl-1d-1 and lipid content 0.018 gl-1d-1 was obtained. The 38.29% lipid content was achieved.
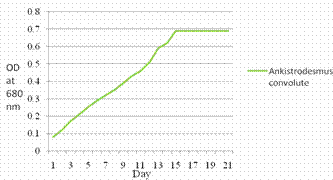
Figure 3. Growth curve of Ankistrodesmus convolutes at the Optical density 680 nm
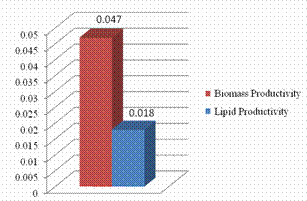
Figure 4. Biomass and lipid productivity (g/l/d) of Ankistrodesmus convolutes
The Chemical conversion of algal oil to fatty acid methyl ester by base catalyzed transesterification method. The reaction is carried out at 60°C for 60 minutes. At room temperature, biodiesel product phase was separated and purified.
The Fourier Transform-Infrared Spectroscopy is the standard method to study the functional group of both reactant and product obtained. The IR Spectrum Ankistrodesmus convolutes oil and biodiesel was shows in Figure 5 and 6. The IR spectrum algae oil (Figure 5) shows the υ (O-H) stretching of hydroxyl group of fatty acids at 3381.22 cm-1, υ as (sp3 C-H) stretching of hydrocarbon at 2923.09 cm-1, υ (C=O) stretching of carbonyl group from fatty acids at 1741.08 cm-1, methylene (CH2) groups have a characteristic bending absorption at 1460.68 cm-1. The methyl δs (CH3) group have characteristic bending absorption is 1376.12 cm-1, the υ (C-O) stretching of alkyl carbon and oxygen from fatty acids are at 1160.02 cm-1 and δr (CH2) bending (rocking) motion associated with four or more methylene (CH2) groups in an open chain of fatty acid occurs at about 721.24 cm-1 while the IR Spectrum Ankistrodesmus convolutes biodiesel (Figure 6) shows the υas (sp3 C-H) stretching of ester group of fatty acid methyl ester at 2922.75 cm‑1, υ (C=O) stretching of ester at 1743.21 cm-1, δs(CH2) bending of methylene group at 1457.85 cm-1, δs(CH3) bending of methyl group absorption frequency at 1366.21 cm-1, υ (C-O) stretching of alkyl carbon and oxygen from fatty acid methyl ester at 1163.15 cm-1, δr (CH2) bending of methylene group was observed at 721.34 cm-1.
The FT-IR analysis is carried out both Ankistrodesmus convolutes algal oil and biodiesel, the hydroxyl group (–OH) of fatty acid is disappear which not observed in IR spectrum of algal biodiesel, the appearance of peak in algal oil and its biodiesel was different.
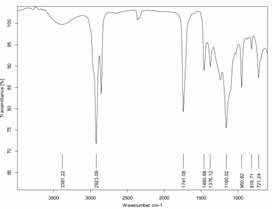
Figure 5. FT-IR spectrum of Ankistrodesmus convolutes oil
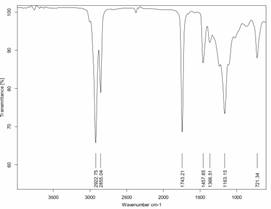
Figure 6. FT-IR spectrum of Ankistrodesmus convolutes biodiesel
Gas Chromatography mass spectroscopy is method used to separate and identify the chemical component of the biodiesel. Gas Chromatography separates the component while Mass Spectroscopy helps to find molecular weight, structure and stereochemistry of molecule.

Figure 7. The qualitative peaks of Gas Chromatography spectrum of Ankistrodesmus convolutes microalgae Biodiesel
Table.1. Ankistrodesmus convolutes biodiesel components with RT, Percentage Area, Name of the Compound (FAME) and Molecular Formula
|
Sr. No. |
Retention Time (min.) |
Area % |
Name of the Compound |
Molecular Formula |
|
1 |
25.389 |
15.36 |
Hexadecanoic acid, methyl ester |
C17H34O2 |
|
2 |
27.461 |
18.59 |
9,12-Octadecadienoic acid (Z, Z) methyl ester |
C19H34O2 |
|
3 |
27.539 |
30.55 |
9-Octadecenoic acid methyl ester |
C19H36O2 |
|
4 |
27.783 |
14.11 |
Octadecanoic acid, methyl ester |
C19H38O2 |
|
5 |
30.005 |
2.05 |
Methyl 18-methylnonadecanoate |
C21H42O2 |
|
6 |
32.986 |
12.93 |
Methyl 20-methyl-heneicosanoate |
C23H46O2 |
The Figure 7 shows the qualitative peaks of Gas Chromatography spectrum of Ankistrodesmus convolutes microalgae Biodiesel. Component of product was separated according to mass to charge ratio. Table 1 show the six types of fatty acid methyl ester components of Ankistrodesmus convolute biodiesel. The chromatogram shows peaks at different retention time with different concentration. The experimental test results and the ester were confirmed with MS library. At the retention time 25.389 min., Hexadecanoic acid, methyl ester (15.36%); at 27.461 min., 9, 12-Octadecadienoic acid (Z,Z) methyl ester (18.59); at 27.539 min., 9-Octadecenoic acid methyl ester (30.55%); at 27.783 min., Octadecanoic acid, methyl ester (14.11%); at 30.005 min., Methyl 18-methylnonadecanoate (2.05%) and at 32.986min., Methyl 20-methyl-heneicosanoate (12.93%) were obtained. The FAME at the retention time 25.389, 27.783, 30.005 and 32.986 shows the base peak at m/z 74.05 while at 27.461 and 27.539 shows the base peak at m/z 81 and 65 respectively. The GCMS data shows that 9-Octadecenoic acid methyl ester possesses highest FAME percentage which was next to follow the 9, 12-Octadecadienoic acid (Z, Z) methyl ester and Hexadecanoic acid, methyl ester. Ankistrodesmus convolutes microalgae Biodiesel was consist of 46.5% saturated FAME and 49.14 % unsaturated FAME.
Conclusions
The microalgae Ankistrodesmus convolutes was green algae belonging from the division Chlorophyta. BBM was used for the growth study of microalgae species. The productivity of biomass was 0.047gl-1d-1 and lipid 0.018 gl-1d-1 was obtained. The chemical conversion of microalgae oil to biodiesel by base catalysed transesterification at 60°C for 60 minutes gave rise to 95.64% of biodiesel friction. The analysis of obtained product was performed by FT-IR and GCMS techniques. The FT-IR shows characteristic ester functional group frequency was appeared at 1743.21 cm-1. The GCMS results the mixture of six type of fatty acid methyl ester. Ankistrodesmus convolutes microalgae biodiesel was the mixture of 46.5% saturated and 49.14 % unsaturated fatty acid methyl ester. The identified fatty acid methyl ester was ranging from C17 to C23. According to Chisti (2007), the saturated and unsaturated fatty acid methyl ester possesses high oxidative stability.
The utilization of microalgae renewable biomass energy in large extent to provides sustainable development of nation which link to global stability, economic balance, innovation in local market, employment and quality of life. Thus, Ankistrodesmus convolutes could be considered as a potential algae for biodiesel production.
References
1. Kulkarni M. G., Dalai A. K., Waste cooking oil-an economical source for biodiesel: A review, Ind. Eng. Chem. Res., 2006, 45, p. 2901-2913.
2. Ministry of Petroleum and Natural Gas, Report of the Working Groups on Petroleum and Natural Gas Sector, XI Plan (2007-2012); http://planningcommission.nic.in/aboutus/committee/wrkgrp11/wg11_petro.pdf (accessed 04/10/2012).
3.
Planning Commission. Government of
4. Klass L. D., Biomass
for renewable energy, Fuels and Chemicals, Academic Press,
5. Ramachandra T. V., Mahapatra D. M., Karthick B., Gordon R., Milking diatoms for sustainable energy; biochemical engineering vs gasoline secreting diatoms solar panels, Ind. Eng. Chem. Res., 2009, 48, p. 8769-8788.
6. Shay E. G., Diesel fuel from vegetable oils: Status and Opportunities. Biomass Bioenergy, 1993, 4, p. 227-242.
7. Abe K., Nishumura N., Hirano M., Simultaneous production of β-carotene, vitamin E and vitamin C by the aerial microalga Trentepohia aurea., J. Appl. Phycol., 1999, 11, p. 33-36.
8. El-Baz F. K., Aboul-Enein M. A., El-Baroty, G. S. Youssef, A. M., Abd El-Baky H. H., Accumulation of antioxidant vitamins in Dunaliella sallina, Online J. Biol. Sci., 2002, 2, p. 220-223.
9. Abd El-Barky H. H., Moawd A. El-behairy A. N., El-Baroty G. S., Chemoprevention of benzo[a]pyrene induced carcinogen and lipid peroxidation in mice by lipophilic algae extract (phycotene), J. Med. Sci., 2002, 2, p. 185-193.
10. Phukan M. M., Chutia R. S., Konwar B. K., Kataki R., Microalgae Chlorella as a potential bio-energy feedstock. Appl. Energy, 2011, 88, p. 3307-3312.
11. Chisti Y., Biodiesel from microalgae, 2007, Biotechnol Adv., 2007, 25, p. 294-306.
12. Singh R., Behera S., Yadav Y. K., Kumar S., Potential of wheat straw for biogas production using thermophiles, in Recent Advances in Bio-Energy Research, Eds S. Kumar, A. K. Sarma, S. K. Tyagi, and Y. K. Yadav (Kapurthala: SSS-National Institute of Renewable Energy), 2014, p. 242-249.
13. Demirbas A., Use of algae as biofuel sources. Energy Convers. Manag., 2010, 50, p. 14-34.
14. Bligh E. G., Dyer W. J., A rapid method of total lipid extraction and purification. Can J Biochem Physiol Pharmacol., 1959, 37, p. 911-917.
15. Bisen P. S., Sanodiya B. S., Thakur G. S., Baghel R. K., Prasad B. K. S., Biodiesel production with special emphasis on lipase-catalysed transesterification. Biotechnol. Lett., 2010, 32, p. 1019-1030.
16. Surendhiran D., Vijay, M., Microalgal biodiesel-acomprehensive review on the potential and alternative biofuel, Res. J. Chem. Sci., 2012, 2, p. 71-82.
17. Dalvi S. N., Sonawane S. R., and Pokharkar R. D., Preparation of Biodiesel of Undi seed with In-situ Transesterification. Leonardo Electronic Journal of Practices and Technologies., 2012, 20, p. 175-182.
18. Meher L. C., Vidya S. D., Naik S. N., Technical aspects of biodiesel production by transesterification – a review, Renew. Sustain. Energ. Rev., 2006, 10, p. 248-268.