Investigation of the effect of antioxidant extract from orange peel on lipids oxidation
Uduak George AKPAN*, Eyitayo Amos AFOLABI, and Martins Onuche ADEJOH
Department of Chemical Engineering, Federal University of Technology, Minna, Nigeria
E-mails: *ugaekon@yahoo.co.uk; elizamos2001@yahoo.com
* Corresponding author, phone: +234 8068 2653 08
Abstract
This research involved investigation of the extraction yield and antioxidant property of orange peel on lipid oxidation. Orange peel was oven dried, grinded to powder and extraction procedure carried out using methanol as solvent in a soxhlet extractor. The effects of time and temperature on the extraction process were considered and results obtained showed an optimum extraction temperature and time of 50ºC and 120 minutes respectively. X-ray fluorescence analysis of the orange peel extract showed that potassium and calcium are the major elements by percentage composition of 55.5 and 32.65 respectively. In studying the effects of orange peel extract on the melon oil sample, peroxide, free fatty acid and pH analysis were carried out for a period of 60 day. The results obtained confirmed the ability of orange peel extract as antioxidant agent.
Keywords
Antioxidant; Orange peel; Melon oil; Oxidation; Rancidity
Introduction
Orange peel, a major by-product of the orange processing industry is often discharge unprocessed, thereby constituting a major source of pollution to the environment [1]. Many studies have been carried out to evaluate the extent [2] and abatement [3, 4] of pollution in the environment. The present study is focusing at converting the seemingly waste (orange peel) into a useful compound, thereby ridding the environment of pollution. The sweet orange (citrus sinensis) is a member of the citrus family, along with mandarins, lemons, limes, grapefruit, and kumquats. Fruits and vegetables are the major sources of natural antioxidants and contain various types of antioxidant compounds such as carotenoids, lycopene, lutein, vitamin E and vitamin C [5, 6].
Antioxidants are the chemical compounds with the ability to delay or prevent the destructive process of oxidation [7]. They act as radical scavengers which slowdown oxidation and convert the radicals to less reactive kind. Antioxidants can be categorized as preservatives as they are used to prevent reaction of some foods constituents (primarily fat and oil or foods of animal origin, such as egg) with oxygen [8]. An unsaturated fat are easily attacked by oxidation process and eventually makes it rancid. In food and diet, antioxidants prevent the formation of free radicals and peroxides; and these are compounds that damage cell’s structure and possibly result in cancer [9, 10]. The applications of antioxidants are widespread in the food industry and are used in lubricant to prevent sludge formation, synthetic and natural pigments from discoloration, preventing polymers from oxidative degradation, feedstuffs, beverages and baking products, as well as dietary supplements [11, 12].
Hegazy and Ibrahim [1] evaluated the efficiency of different organic solvents for extraction of flavonoids and polyphenolic compounds from orange peels. They observed that methanol and ethanol were the best solvents for the extraction of this plant constituent. This research is aimed at the extraction and characterization of antioxidant from orange peel at different temperature and time using soxhlet extraction process; ultimately focusing at testing the ability of the antioxidant on lipid oxidation. In addition, the X-ray fluorescence analysis of the orange peel extract was carried out and the effect of the extract on melon seed oil sample investigated.
This research will go a long way in promoting the extraction of antioxidant from orange peels at minimum cost. In addition, it will complement the efforts of existing factories toward meeting the rising demand of antioxidant.
Materials and method
Orange peels were collected from Orange sellers at Bosso Market, Niger State, Nigeria. The glass wares and some of the equipments used in this work were provided by the Chemical Engineering Department Laboratories of Federal University of Technology, Minna and Cereals Research Institute, Bida, Niger State, Nigeria.
The wet and fresh peels were weighed using digital weighing balance to obtain its initial weight. It was oven-dried for 48 hours at 40°C and 5 g in triplicate of the peel was used to determine the moisture content. The dried orange peel was milled into a fine powder of 0.5 mm particulate size by a Model ED 5, Thomas Wiley milling machine at 4000 rpm. The dried grinded orange peel was afterward wrap up into low-density polyethylene and stored for extractions [1].
A known amount (20 g) of the sample was weighed and extraction of orange peel was performed in a soxhlet extractor using 200 ml of methanol. The sample was extracted at a time of 60 minutes for various temperature ranges of 20, 30, 40, 50 and 60°C. In the same manner, 20 g of the sample was weighed and mixed with 200 ml methanol using the solvent-to-solid ratio of 10:1. The sample was extracted at 50ºC for various time ranges of 20, 40, 60, 80, 100, 120, 140, 180 minutes. After extraction, the extracted sample was filtered through filter paper and evaporated using a rotary evaporator. The evaporated sample was then prepared for X-ray fluorescence analysis in order to determine the major and minor elements present in the orange peel extract.
Evaluation of lipid oxidation of orange peel extract
The evaluation of the antioxidant property of the orange peel extract was carried out by lipid oxidation test on an oil sample. The oil sample was prepared by mixing unsaturated melon seed oil with saturated animal fat in the ratio of 4:1 and 5 ml of orange peel extract was added to 40 ml of oil. The peroxide value, acid value and pH tests were used to analyze antioxidant effectiveness of the orange peel extract on a mixture of melon seed oil and animal fat. In addition, Lipid oxidative deterioration was checked on the oil. The peroxide value and acid value tests were carried out according to the standard procedures stated in Etti et al. [13], John [14] and Fereidoon and Ying [15].
The percentage of antioxidant effectiveness was calculated using the method described by Adegoke and Gopalakrishna [16] from the equation (1).
|
AE (%) = (PVC – PVT)/PVC |
(1) |
where AE is the antioxidant effectiveness, PVC is the peroxide value of control sample and PVT is the peroxide value of test sample.
Results and Discussion
Effect of time on extraction yield of orange peel
Effects of different extraction time were investigated to obtain the optimum time in the extraction of orange peel. As shown in Figure 1, the extraction yield of orange peel increased with extraction time from 20 to 120 minutes. Thereafter, the yield slowly reaches a constant after 150 minutes. However, 120 minutes is the optimum time for the extraction as 0.5% addition in yield is so negligible compared to 30 minutes reaction at 50°C required to have a yield of 47% from 46.5%. Between 120 and 180 minutes, the extraction yield was almost constant. This mean, all the orange peels are not fully solubilised in the solvent. Therefore, the extraction yield gradually increased until 120 minutes.
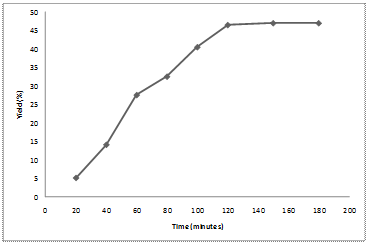
Figure 1. Time effects on percentage yield of orange peel extract
Effect of temperature on extraction yield of orange peel
Effects of different extraction temperatures at 60 minutes on extraction yields was determined and the results shown in Figure 2. Temperature greatly influenced the extraction yield; as at 20°C, just about 1% extract was realized. However, a great increase in the extraction yield was noticed as temperature increases from 20°C to 50°C. Furthermore, an increase in temperature to 60°C resulted in a very small increase in the percentage yield. An optimum temperature of 50°C is selected as the energy to be expended at 60°C compared to the amount of yield enhancement between 50°C and 60°C is considerably too high. Therefore, temperature of 50°C is adjudged the best temperature of extraction as the yield at this temperature is considered the highest when compared with the yields at other extraction temperatures.
This result is in agreement with the research work of Borang [17], who observed that an increase in temperature enhances solvent extraction of the antioxidant compounds thereby improving both diffusion coefficients and the solubility of a substance. In addition, this result showed that extraction temperature is an important factor to be considered in improving the efficiency of the extraction.
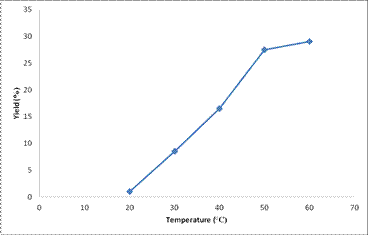
Figure 2. Temperature effects on percentage yield of orange peel extract
X-ray Fluorescence analysis of orange peel extract
X-ray fluorescence analysis was performed in order to find out the major and trace elements present in the orange peel extract sample and the results obtained are presented in Table 1. Potassium (K) and Calcium (Ca) are the major elements contained in the antioxidant as their compositions are found to be 55.5 and 32.65 % respectively. However, elements such as Phosphorus (P), Sulphur (S), Magnesium (Mg), Zinc (Zn) and iron (Fe) are classified as intermediate and elements such as Copper (Cu), Chlorine (Cl), Nickel (Ni), Titanium (Ti) and Sodium (Na) were found to be the minor elements based on their percentage compositions.
The results are in agreement with the results of the findings of Gaitry et al. [18], Borang [17], Skykorova et al. [19]. They also reported that K and Ca had the highest percentage composition in the extract. These compositions proved that K and Ca are the major elements present in most antioxidants. For example, Gaitry et al. [18] reported that 66% potassium content is present in Glycyrrhiza Glabra root extract. Although, the antioxidant produced in this work is majorly made up of metallic elements, it is important to note that elements considered toxic, even at low level were absent in the analysis of the antioxidant extracted from orange peel. Therefore, the antioxidant obtained in the present study is safe for use. The methanol extract of the orange peel showed the presence of important metals like Ca, K, Fe and Mg which are beneficial for the metabolism [18].
Table 1. X-ray fluorescence analysis of orange peel extract
|
Elements present |
S |
P |
Cl |
K |
Ti |
Fe |
Ni |
Cu |
Zn |
Re |
Ca |
Na |
Mg |
Mn |
|
Orange peel extract (%) |
1.0 |
2.3 |
0.67 |
55.8 |
0.41 |
1.89 |
0.36 |
0.50 |
1.1 |
1.1 |
32.65 |
0.02 |
1.3 |
1.0 |
Effect of Peroxide value test on melon seed oil
Peroxides are the products formed at the initial stages of lipid oxidation and therefore, their values can be used as a measure of how lipid oxidation occurs. In investigating the effect of orange peel extract on the melon seed oil, there is a decrease in the peroxide value of the sample without orange peel extract in comparison with the sample mixed with orange peel extract. For example, peroxide value of samples without and with orange peel extract decreases from 6.09 to 6.05 at day 1. The same pattern is observed when comparing the peroxide values of samples without and with orange peel extract for a period of 30 and 60 days. The control sample is observed to have higher peroxide value as it did not contain orange peel extract.
As shown in Table 2, lipid oxidation was observed to be slower in melon sample mixed with orange peel extract. This confirmed that the active component of the orange peel is responsible for the anti-oxidization of the oil sample. The result obtained is in conformity with the study of Etti et al. [13] on the use of antioxidants to minimize rancidity in mayonnaise and their results showed that samples with Aframomum danielli extracts had effects on their samples compared to samples without any antioxidants.
Table 2. Effect of Peroxide value test on oil sample
|
Sample |
Day 1 |
Day 30 |
Day 60 |
|
Sample with orange peel extract (meq.g/kg of oil ) |
6.05 |
6.20 |
6.45 |
|
Sample without orange peel extract(control) (meq.g/kg of oil ) |
6.09 |
6.40 |
6.85 |
|
Antioxidant Effectiveness (%) |
0.66 |
3.125 |
5.84 |
The antioxidant effectiveness as shown in Table 2 is 0.66 % at day 1 and increases to 3.125 % and 5.84 % at day 30 and 60 respectively. This means, lipid oxidation is smallest at the initial stage and increased with increase in days of storage.
Effect of free fatty acid test on oil sample
The results of changes in the values of free fatty acid of oil samples with and without the orange peel extract are tabulated in Table 3.
Table 3. Effect of free fatty acid test on oil sample for a 60 day period
|
Sample |
Day 1 |
Day 30 |
Day 60 |
|
Sample with orange peel extract (mgKOH/1g of oil) |
3.90 |
3.95 |
4.10 |
|
Sample without orange peel extract(control) (mgKOH/1g of oil) |
3.93 |
4.10 |
4.30 |
The value of free fatty acid of melon seed oil samples with orange peel extract is lower when compared with the sample without orange peel extract. The same trend was observed for samples at the end of 30 and 60 days. This confirmed that addition of orange peel extract to the melon sample reduces the formation of free fatty acid and indirectly rancidity. This result further affirmed that orange peel extracts was able to reduce rancidity. The result obtained in this study is in agreement with the work of Etti et al. [13], who observed that rancidity is often accompanied by FFA formation.
Effect of pH test on melon seed oil
Generally, there is a decrease in pH of melon seed oil with increase in the days of storage of the sample without orange peel extract in comparison with that of the sample with orange peel extract and this reduction moved towards acidic region (Table 4).
Table 4. Effect of pH test on oil sample
|
Sample |
Day 1 |
Day 30 |
Day 60 |
|
Sample with orange peel extract |
5.30 |
5.30 |
5.25 |
|
Sample without orange peel extract(control) |
6.00 |
5.95 |
5.86 |
The high pH of the sample without orange peel extract showed the absence of antioxidant to reduce the activities of enzymes and microorganisms. However, the difference in the pH value of samples without and with extract increases with increase in days. This result confirms the presence of antioxidants in orange peel as its extract was found to inhibit the activities of enzymes and microorganisms.
Conclusion
Antioxidant which has the ability to preserve melon seed oil from deterioration for 60 days was extracted from orange peel. The optimum extraction temperature and time were 50ºC and 120 minutes respectively. The effect of orange peel extract on oil sample showed that the presence of antioxidant in orange peel is capable of preventing lipid oxidation and prolonging the shelf life of fat and oil food stuffs.
References
2. Ndoke P. N., Akpan U. G., Kato M. E., Contributions of Vehicular Traffic to Carbon Dioxide Emissions in Kaduna and Abuja, Northern Nigeria, Leonardo Electronic Journal of Practices and Technologies, 2006, 5(9), p. 81-90.
3. Akpan U. G., Hameed B. H., Solar degradation of an azo dye, acid red 1, by Ca–Ce–W–TiO2 composite catalyst, Chemical Engineering Journal, 2011, 169(1), p. 91-99.
4. Akpan U. G., Hameed B. H., Photocatalytic degradation of wastewater containing acid red 1 dye by titanium dioxide: effect of calcination temperature, Desalination and Water Treatment, 2012, 43(1-3), p. 84-90.
5. Helio F., Nuno G., Cecilia B., Ana P. D., Antioxidant Activity of Lignin Phenolic Compounds Extracted from Kraft and Sulphite Black Liquors, Molecules, 2010, 15(12), p. 9308-9322.
7. Antioxidants. Available at: http://en.wikipedia.org/wiki/antioxidants (accessed 15/08/2013)
9. Guo C., Yang J., Wei J., Li Y., Xu J., Jiang Y., Antioxidant activities of peel, pulp and seed fractions of common fruits as determined by FRAP assay, Nutrition Research, 2003, 23, p. 1719-1726.
10. Kanner J., Rosenthal I., An assessment of lipid oxidation in foods, Journal of International Union of Pure and Applied Chemistry, 2000, 64(12), p. 1959-1964.
15. Fereidoon S., Ying Z., Lipid oxidation: Measurement methods in Bailey’s Industrial Oil and Fat Products, Canada, John Wiley and Sons, 2003.
17. Rejal S. Z. B., Extraction of antioxidant, phenolic content and mineral content from Guava peel, B.Sc Thesis, Faculty of Chemical & Natural Resources Engineering, Universiti Malaysia, Pahang, 2010.