Corrosion behaviour of some conventional stainless steels in electrolyzing process
Amal NASSAR*and Eman NASSAR
Department of Mechanical Engineering, Higher Technological Institute, Tenth of Ramadan City, Egypt
E-mail: amal.nasser@hti.edu.eg
Corresponding author: Phone: +201006414107; Fax: +20224924319
Abstract
In this study, attempts were made to increase the amount of hydrogen generated from the water electrolysis process. Some conventional stainless steels (316; 409; 410 and 430) were used as anode and cathode in electrolysis process. Further study was carried out on the corrosion trend in all the investigated metals. It is observed that the electrode material can effect on the amount of hydrogen generate by electrolyzing process and metal composition of the stainless steels effects on the rate of corrosion.
Keywords
Corrosion behaviour; Stainless steels; Electrolyzing
Introduction
Over the past years, it is becoming more likely that the emphasis on cleaner fuel will lead to the use of hydrogen in a significant way. Hydrogen is a chemical element with chemical symbol H and atomic number 1 with an atomic weight equal 1.00794, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the universe unlike oxygen, hydrogen is not found as free in the nature at any significant concentration, constituting roughly 75% of all baryonic mass. Hydrogen is a first element on the periodic table which making it the lightest element on the earth. Hydrogen gas is too light, it can rise in the atmosphere and it is rarely found in its pure form H2 [1].
Hydrogen fuel is a zero-emission fuel, which uses electrochemical cells, combustion in internal engines, to power vehicles and electric devices. Hydrogen fuel is also used in propulsion of the spacecraft and can potentially be mass produced and commercialized for the passenger vehicles and for the aircraft [2]. Hydrogen energy is an environment friendly. Because of the actual human ecological concerns, the exploitation of hydrogen as a universal fuel would be greatly acclaimed. During last two decades or so, the elaboration of the hydrogen based economy has made important progress on the account of the numerous research projects such as the hydrogen fuel cell and hydrogen car [3].
Hydrogen is produced using both renewable and non-renewable resources, through various process routes. The available technologies for hydrogen production are reforming of natural gas; gasification of coal and biomass; and the splitting of water by water-electrolysis, photo-electrolysis, photo-biological production, water splitting thermo chemical cycle and high temperature decomposition. The principal methods for the production of hydrogen involve water-electrolysis and natural gas reforming processes [4]. The worldwide increasing demand of hydrogen, such as in hydrogen fuel cells, has made it crucial to find hydrogen generation methods from inexpensive simple processes, many researchers suggested some innovative routes. Interestingly, a large number of the methods included sodium hydroxide as an essential ingredient. The use of sodium hydroxide for production of hydrogen is not new and was in an application even during 19th century [5]. Metallic aluminium has a high potential with a large chemical energy Therefore, the purpose of this study is to find the suitable stainless to the Water electrolysis process.
Factors effect on the efficiency of the electrolyzing
Electrolyte quality: Bases and acids have a great reducing effect on the overvoltage value of an electrolyser [6, 7] due they improve the ionic conductivity aqueous electrolyte compounds. The concentration levels of these solutions are limited in practice due to the highly corrosive behaviour of such materials. A 25% to 30% KOH aqueous solution is reported to have a wide use in electrolysers [8]. Also, the electro catalytic performance of water electrolysis cell is known to be limited [9, 10]. This limitation is mainly cause the overall electrical resistance of a cell to rise and will reduce the efficiency. Therefore, substitute electrolytes such as; ionic liquids have been introduced to improve the conductivity and stability factors of electrolytic baths [11, 12]. De Souza [13] performed a series of experiments on the use of the ionic liquid of in water at ambient temperature. The electrode plates of these cases were selected from a number of easily found metals such as carbon steel; Nickel; Nickel Molybdenum alloy and Molybdenum. A maximum efficiency value of 96% was reported for the case of low carbon steel electrodes [14] in 10 vol. % aqueous solution of MBI. MF4. All tests took place at the current density value of 44mA cm-2.
Temperature: Electrolysis process is much more efficient at high temperatures [14]. The reasons of this behaviour can be discussed according to the thermodynamic characteristics of a water molecule. On other hand, ionic conductivity and surface reaction of an electrolyte rise directly with temperature [15]. Electrolysis Water at High temperature requires less energy to reach any given current density in analogy with a low temperature process [16, 17]. As a practical example of the latter, Bailleux [18] tracked the operation of a test hydrogen production plant for 2 years. The final report shows a voltage reduction of 120 mV as the temperature was raised from 120°C to 150°C. On the other hand, the increased temperature and pressure were mentioned to cause some stability problems such as container cracks and gasket leaks.
Electrode material: A wide range of materials are being used as electrodes. Each metal has a different level of activity; electrical resistance and corrosion resistivity. Gold and platinum are known to be two of the best choices for being used as electrodes. However, high prices limit their usage in industrial and commercial electrolysers. Aluminium, Raney nickel, Nickel and cobalt are the most common electrode materials for being used in alkaline electrolytic baths. This popularity is the result of their satisfactory price range, corrosion resistance and chemical stability [19].
Appleby [20] conducted a set of experiments by utilizing different electrodes such as 99.99% pure Ni, Ir, Pt and Rh as well as Ni cloth, Ni-Cd, Ni sinter and low impregnation Nickel and cobalt moly date catalyst on nickel sinter. The results show that nickel gives more desirable potential characteristics among the mentioned materials. Also, authors found woven or porous sintered electrodes to be thirty times more active those with a smooth surface. Electrode with large surface area causes an apparent exchange current density. The latter is known to be the main reason of the excess observed activity. There is a wide range of variations in the value of electrode-electrolyte activity for different materials. For example, platinum electrodes show higher activity levels in contact with KOH aqueous solutions in comparison with molybdenum plates [12].
Material and method
Water electrolysis process needs a material with a good current connection (conductivity) and good resistance to corrosion. We select stainless steel TYPE 316; stainless steel TYPE 409; stainless steel TYPE 410 and stainless steel TYPE 430. Table 1 shows the chemical composition of each type.
Table 1. Chemical composition of each stainless steel type
|
Grade |
% from |
||||||||
|
C |
Mn |
Si |
P |
S |
Cr |
Mo |
Ni |
N |
|
|
316 |
0.08 |
2 |
0.75 |
0.045 |
0.03 |
17 |
3 |
13 |
0.1 |
|
409 |
0.08 |
1 |
1 |
0.045 |
0.03 |
10.75 |
0 |
0.5 |
0.75 |
|
410 |
0.15 |
1 |
1 |
0.04 |
0.03 |
12 |
|
25 |
|
|
430 |
0.12 |
1 |
1 |
0.04 |
0.03 |
17 |
|
0.75 |
|
Water electrolysis
A direct current (DC) is applied to maintain the electricity balance and electrons flow from the negative terminal of the DC source to the cathode at which the electrons are consumed by hydrogen ions (protons) to form hydrogen. In keeping the electrical charge (and valence) in balance, hydroxide ions (anions) transfer through the electrolyte solution to anode, at which the hydroxide ions give away electrons and these electrons return to the positive terminal of the DC source. In order to enhance the conductivity of the solution, electrolytes which generally consist of ions with high mobility are applied in the electrolyzing [21].
A water electrolysis process made by using pipes with diameter 19 mm (0.748 inch) and length 170 mm (6.69 inch) as shown in figure 1 These pipes will be used as anode and 5 pipes with diameter 22 mm (0.866 inch) with length 150 mm (5.9 inch) pipes will be in larger diameter pipes to 5 cells as shown in figure 2. All pipes were put in PVC pipe with inner diameter 110 mm and thickness 5 mm and we powered the overall cell with (12 V) DC power supply (8 A), and we connected the 5 cells in parallel so that the voltage on one cell (12 V) distance between the pipes in one cell is (1.5 mm).
a. b.
b.
Figure 1. Stainless steel pipes (a. side view, b. top view)

Figure 2. Anode& cathode in PVC pipe
Results and discussion
Measuring corrosion rate
The corrosion rate of stainless steel in the test solutions (NaCl) is calculated from decrease in weight observed in samples in weight loss tests using following formula [22].
Corrosion rate (mpy) = 534 w/ DAT
where: mpy: mils penetration per Year, W= weight loss (mg)
D= density of sample g/cm3
A= area of the sample (inch) = 2πr2 + 2πrh
T= exposure time (hrs.)
Measuring gas flow rate
To measure the gas flow rate, a simple experiment was done to measure the flow rate of gas output for each kind of stainless steel from a single cell. Figure 3 illustrates the flow rate test.
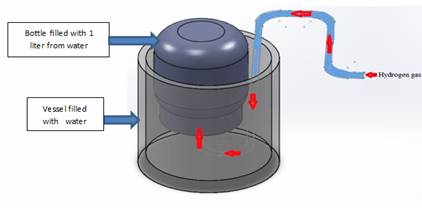
Figure 3. The flow rate test
The stainless steel specimens were taken out of PVC tube after running for 3, 6, 9, 12, 15, 18, 21, 24, 27 and 30 days in the electrolyzing cell. The inert and outer pipes were weighed. Weight changes in the range of 0.0 31 to 0.027 Kg was noted, indicating very nominal changes during electrolyzing process exposures. The corrosion rate values computed from weight losses have the range of 31.78 to 2.88 mpy. Figures 4, 5 show the weight loss for each stainless steel type. Figures 6-9 show the relationship between running time and corrosion rate. Figure 10 illustrates the effects of the electrolyzing process on the surface of each stainless steel type where corrosion pitting occurred with different sizes and densities.
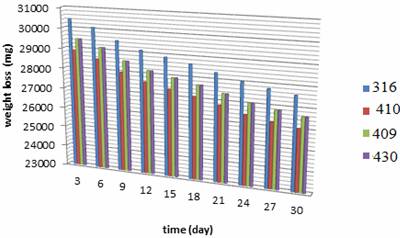
Figure 4. Weight loss during the electrolyzing process for inert rod
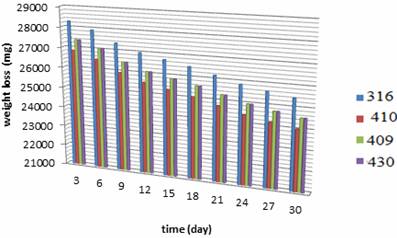
Figure 5. Weight loss during the electrolyzing process for outer rod
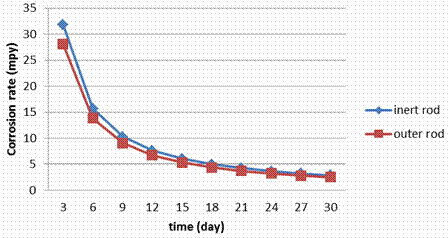
Figure 6. Corrosion rate during the electrolyzing process for S.S 316 rod
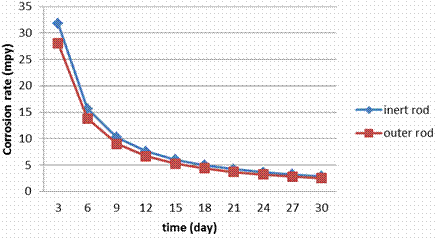
Figure 7. Corrosion rate during the electrolyzing process for S.S 409 rod
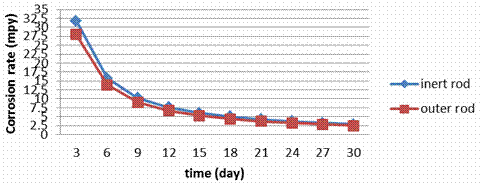
Figure 8. Corrosion rate during the electrolyzing process for S.S 410 rod
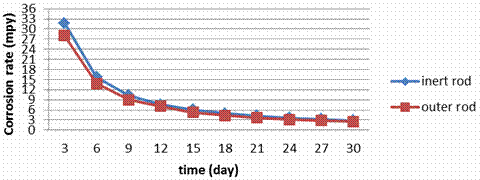
Figure 9. Corrosion rate during the electrolyzing process for S.S. 430 rod
The corrosion rate data (Figure 9) for stainless steels in electrolyzing cell indicate that the rates are greatly dependent on the composition of the steels particularly on Cr, Mo and Ni contents. No changing in the corrosion rate for the inner rod and outer rod in the electrolyzing cell this is because all surfaces are exposed to the similar conditions. Figures show also that the corrosion rate in S.S 409 and 430 are too close; this is because both of them have small amounts of Ni (0.5 to 0.75).
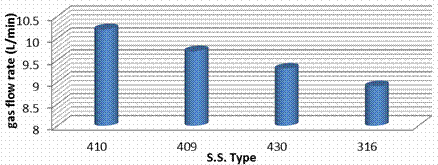
Figure 10. Gas flow rate for each stainless steel type
Figure 10 shows gas flow rate for each stainless steel type. The flow rate measurement shows that all stainless give a satisfactory result (10.2 to 9.2 L/min). This is because Electrical conductivity for each stainless steel type is similar (78. µΏ cm) but S.S 410 gives high flow from hydrogen this is because it have large amount of Nickel. The same result was indicted by Becker et al. [23].
Conclusion
This work shows that the material of the electrode can effect on the amount of hydrogen generate by electrolyzing process. Corrosion rates of conventional stainless steels in the electrolyzing process are extremely low, but the latter has significantly higher corrosion rates than the conventional steels. Nickel plays a decisive role in determining the corrosion rate in stainless steel in the electrolyzing process. The hydrogen generation rate for Stainless Steel 410 was high compared with other stainless steels.
References
1. Palmer D., Hydrogen in the Universe, NASA, 2008, p. 30.
2. Altork L. N., Busby J. R., Hydrogen fuel cells: part of the solution, Technology & Engineering Teacher, 2010, 2, p. 22-27.
3. Andersen E. R., Frederikstad N. O., Method for producing hydrogen, Patent Application Publication, United States, 2003, p. 3-7.
4. Probstein R. F., Hicks R. E., Synthetic fuels, 3rd Ed. New York, Dover Publications, 2006.
5. Kumar S., Surendra K., Saxena Role of sodium hydroxide for hydrogen gas production and storage, Materials and processes for energy: communicating current research and technological developments, FORMATEX, 2013, p.71-76.
6. Millet P., Andolfatto F., Durand R., Design and performance of a solid polymer electrolyte water electrolyzer, International Journal of Hydrogen Energy, 1996, 21, p. 87.
7. Badwal S. P. S., Giddey S., Ciacchi F. T., Ionics, Hydrogen and oxygen generation electrolyte membrane (PEM)-based electrolytic technology, New York: Plenum Press, 2006, p. 7-11.
8. Guttman F., Murphy O. J., Water electrolysis with inductive voltage, Pulses In book: R.E. White, J.O'M. Bockris, B.E. Conway, Modern Aspects of Electrochemistry. New York: Plenum Press, 1983, p. 513-520.
9. Crabtree G. W., Dresselhaus M. S., Buchanan M. V., The hydrogen economy physics today, 57, 2004, p. 39-48.
10. Robert S. A., Osteryoung voltammetric determination of water in an aluminum chloride-N-n-butylpyridinium chloride ionic liquid, Analytical Chemistry, 1983, 55, p 1970-1973.
11. De Souza R. F., Padilha J. C., Gonçalves R. S., Dupont J., Room température dialkylimidazolium ionic liquid-based fuel cells, Electrochemistry Communications, 2003, 5, p. 728–731,
12. de Souza R. F., Loget G., Padilha J. C., Martini E. M. A., de Souza M. O., Electrochemistry Communications, Molybdenum electrodes for hydrogen production by water electrolysis using ionic liquid electrolytes, 2008, 10, p. 1673.
13. De Souza R. F., Padilha J. C., Gonçalves R. S., Rault-Berthelot J., Dialkylimidazolium ionic liquids as electrolytes for hydrogen production from water electrolysis, Electrochemistry Communications, 2006, 8, p.211.
14. Nikolic V. M., Tasic G. S., Maksic A. D., Saponjic D. P., Miulovic S. M., Marceta M. P., Kaninski, Raising efficiency of hydrogen generation from alkaline water electrolysis – energy saving, International Journal of Hydrogen Energy, 35(22), 2010, p. 12369-12373.
15. Udagawa J., Aguiar P., Brandon N. P., Hydrogen production through steam electrolysis: Control strategies for a cathode-supported intermediate temperature solid oxide electrolysis cell, Journal of Power Sources, 2007, p. 93-103.
16. Stojic D. L., Marceta M. P., Sovilj S. P., Miljanic S. S., Hydrogen generation from water electrolysispossibilities of energy saving, Journal of Power Sources, 2003, 118(1), p. 315-319.
17. Bockris J. O. M., Conway B. E., Yeager E., White R. E., Theory of the structure of ionomeric membranes, in Comprehensive Treatise of Electrochemistry, Comprehensive Treatise of Electrochemistry. New York, Plenum Press, 1981, p. 44-48.
18. Bailleux C., Advanced water alkaline electrolysis: a two-year running of a test plant, International Journal for Hydrogen Energy, 1981, p. 461.
19. Mingyi L., Bo Y., Jingming X., Jing C,. Thermodynamic analysis of the efficiency of high-temperature steam electrolysis for hydrogen production, J. Power Sources, 2008, 177, p. 493-499.
20. Ganley J. C., High temperature and pressure alkaline electrolysis, International Journal for Hydrogen Energy, 2009, p. 3604-3611.
21. Oldham K. B., Myland J. C., Polarization inventory diagrams, Journal of Electro analytical Chemistry, 2001, 504, p. 137-145.
22. Orubite Okorosaye K., Oforka N. C., Corrosion inhibition of Zinc on HCl using Nypa fruticans Wurmb extract and 1,5 Diphenyl Carbazone, Journal of Applied Sciences and Environmental Management, 2004, 8(1), p. 56-61.
23. Becker M. D., Garaventta G. N., Visintin A., Pulse-current electrode position for loading active material on nickel electrodes for rechargeable batteries, ISRN Electrochemistry, 2013, p. 2-7.