Investigation of the physical properties of tiles produced with Otukpo clay
David Onoja PATRICK*, Haruna Mavakumba KEFAS, Yakubu Mandafiya JOHN,
and Victor Idankpo AMEH
Chemical Engineering Department, Modibbo Adama University of Technology, Yola, Nigeria
E-mails:*dopatrick@mautech.edu.ng, hmkefas@yahoo.com, anaaticha@yahoo.com, idankpo@googlemail.com
* Corresponding author, phone: +2348052727273
Abstract
The study focuses on the production of floor tiles using local clay from Otukpo, Nigeria by plastic forming and properties of the tiles such as absorption, acid resistance, bulk density, compressive strength, and colour change, modulus of rupture, plasticity, shrinkage and thermal shock resistance were investigated. Different samples of tiles were formulated by blending the clay with feldspar, quartz, grog and filler. The compressive strength and modulus of rupture were found to be highest, 482 kg/cm2 and 487.5 kg/cm2 respectively for the clay tile sample. It bulk density was 2.31kg/cm3 and water absorption was 3%. The shrinkage of the clay sample was 16%, however with clay body of 40% clay, 20% feldspar and 40% quartz, it dropped to 6%. All the samples show good resistance to acid attack, thermal shock and have good plasticity. The tile properties showed that they could be used as floor or wall tiles. They could also be used for quarry tiles.
Keywords
Floor tiles; Physical properties; Compressive strength; Clay; Otukpo
Introduction
Ceramic tiles are made of clay, feldspar and flint or quartz [1-3]. The quantity and the quality of the clay that ceramic tile contains play a key role in the final properties of the materials. The properties of the tile can be improved by varying the composition of the main ingredients in the formulation of tiles [4-6]. Ceramic tiles are one of the most durable, long-lasting and attractive flooring materials available. They are formed and fired at a high temperature until they are hardened. The high firing temperature and time results in harder and less porous the tile, resulting in a good quality tile [7]. The impetus for the many current development in ceramics has risen from the critical demands of many industries for improved materials. Particularly, stringent requirement have come from the construction industry where durability, resistance (to corrosion, abrasion, chemicals), strength and water absorption properties are critical [8].
The application of ceramic tiles includes their use in building surfaces, and pipes. Ceramic tiles can be (and are) used in every part of a house – on walls, floors, ceilings, roofs, fireplaces and as an exterior cladding on buildings. Ceramics are much more resistive to changes in temperature than other natural material. This makes ceramic tiles floor to remain cool. They are also easy to clean and do not suffer any damage from bleach, disinfectant or scouring agents. The only seen disadvantages are that ceramic tiles can crack if heavy objects fall on them and sometimes they can be slippery and too cold.
The objective of this work is to determine the physical properties of tiles produced from different formulations of clay, feldspar and quartz and to determine the chemical composition of the clay.
Materials and methods
The materials used for this research work include clay, feldspar, flint, quartz and rice husk (fillers).
Sieved analysis of clay
This was carried out using standard hydrometer method developed based on the work of Bouyoucos [9]. Fifty grams (50.0 g) of freshly dry clay was weighed and 50 ml of 5.0 % hexameta-phosphate alongside 100 ml of distilled water was added. The sample was allowed to stand for 30 minute and then transferred to glass cylinder where water was added to make-up to 1000 ml. The content was shaken and put on a flat surface, immediately soil hydrometer and thermometer were inserted into the suspension. The temperature and 1st hydrometer reading were recorded at 40 seconds. The suspension was allowed to stand for another 2 hours, and then the 2nd hydrometer readings were taken.
The first reading measures the percentage of the sand, i.e.:
|
|
(1) |
The second reading indicates the percentage of 2 micron (total) clay in the suspension:
|
|
(2) |
|
|
(3) |
where H1 and H2 are hydrometer reading and T1 is thermometer reading.
Sample preparation
The dried clay sample was crushed and ground into powder form, using jaw crushers and pulverizing machine. The ground clay was sieved to pass through a sieve of 250 µm aperture size [10]. The feldspar, flint, quartz and rice husk were prepared following the same procedure. The feldspar and quartz samples were washed and calcined before crushing while the rice husk was pulverized without crushing because of its original particle size.
Chemical analysis of clay
The chemical analysis was carried out using energy dispersive X-ray fluorescence, ED XRF method.
Body composition of tile
The tile body composition was formulated by varying the proportion of the clay, feldspar, quartz, grog and sawdust fiber. The body formulation is shown in Table 1.
The various samples were thoroughly mixed. Water was gradually added while working to plastic form. This was continued until desired plastic form was achieved.
Plasticity test
Parts of the plastic mass of samples A to J were taken, and then little ropes about the thickness of 5 mm were made out of it. The coils were then bent into rings of 1 inch diameter. The rings were then observed for cracks [11].
Table 1. Body Formulation of Tile Samples
|
Sample |
Clay (%) |
Feldspar (%) |
Quartz (%) |
Grog (%) |
Fibre (%) |
|
A B C D E F G H I J |
100 80 80 60 40 40 50 40 60 60 |
- 20 - 20 40 20 - 20 20 - |
- - 20 20 20 40 - - - - |
- - - - - - 50 40 - 20 |
- - - - - - - - 20 20 |
Shrinkage test
Five tile bars of 14 cm long, 4 cm wide and 1 cm thick were made from a well kneaded mass of clay of average modeling constituency. Marks of 10 cm length were scratched on the faces of each bar. The tiles were then oven dried at 110 °C. Drying shrinkage was determined using equation 4:
|
|
(4) |
The dried clay samples were fired at 900 °C and the scratch marks were measured. The firing shrinkage was calculated using equation 5:
|
|
(5) |
The total shrinkage may be calculated from equation 6:
|
|
(6) |
Color test
The color of the bars green ware was noted by checking against color chart [12]. Also, the color after firing was noted and recorded.
Absorption test
The fired test pieces were weighed, immersed in water and boiled 1 hour. This was allowed to stand in the same water for a further twenty-three hours. It was then removed and the surface water wiped off and weighed. The corresponding percentage absorption of each sample was then calculated and recorded. The absorption was determined using equation 7:
|
|
(7) |
Acid resistance test
The tile pieces were crushed and sieved. 2.0 g was then weighed from the particles retained on the 1 mm screen. The tile particles was then treated with 5 ml of 0.1 M H2SO4 and heated until the acid fumed vigorously. The sample was then allowed to stand for 24 hours and then thoroughly washed with water. It was oven dry (110 – 1150 °C) and then weighed. The procedure was repeated for treatment with 5 ml of concentrated (98 %) H2SO4.
Cold crushing strength
Samples of dimension 14 cm × 15 cm were obtained for each of the formulated tiles and the areas were calculated. They were then individually placed on equipment called form test hydraulic strength testing machine. The value of the force required to crush the brick was obtained from the machine at the first crushing sound from the sample. This was done based on ASTM C-133 [13, 14].
Modulus of rupture
The three point loading scheme was used to determine the modulus of rupture. A constantly increasing load was applied at the midpoint of the sample until failure is observed (based on ASTM C-133). The load was obtained and used to determine the modulus of rupture [14].
Bulk density
The test samples were dried, weighed, immersed and boiled in water for 1 hour to ensure that all open pores become filled. It is then weighed first immersed in the liquid and then air. The bulk volume and bulk density were determined from Equations 8 and 9:
|
|
(8) |
|
|
(9) |
where, Wa = weight of dry test piece, Wb = weight of test piece soaked and suspended in the immersion liquid, Wc = weight of test piece soaked and suspend in air and, D = density of immersion liquid at temperature of test.
Thermal shock resistance
Fired cubes formed from each sample were used for this test. The cubes were fired (after being observed) in a furnace to a temperature of 1000 °C. At this temperature, the cubes were removed from the furnace using a tong and left in open air for 30 minutes. Thereafter, they were observed for signs of crack or failure. The process was repeated three times to see the effect of reaped heating cycles.
Molding of tiles
To make tile shapes, a chunk of moist plastic clay was cut using the cut-off wire and rolled out into a slap. The desired share (square) of the tile was measured on the slap and using the cutting knife the shape was cut from the slap. The tile so formed was then covered and kept in the cupboard for two days to avoid rapid drying of the piece which causes cracking. After this, the piece was opened and allowed to dry properly.
Bisque firing
The dry tiles were loaded in the furnace and heated gradually to 100 °C. The temperature was maintained for 30 minutes (water smoking). The temperature was then raised to 250 °C and allowed to soak for another 30 minutes. The temperature was gradually risen to 800 °C and allowed to soak for 1 hour. The furnace was then switch off and allowed to cool to about 50 °C before the tile was removed.
Glazing
Glaze with the compositions indicated as ‘Glaze 1’, ‘Glaze 2’ and ‘Glaze 3’ below were prepared and applied to the top surface of the bisque fired tiles using a brush. The glaze was allowed to dry properly, and then the tile was fired from 800 °C to 1200 °C using the firing schedule in the bisque firing stage.
|
GLAZE 1: Cream Colour Recipe: 0.19 CaO 0.37 ZnO 0.12 TiO 0.19 K2O 0.15 Na2O Batch: Whiting – 3.7 % ZnO – 10.5 % TiO – 3.4 % Potash Feldspar – 37.1 % Soda Feldspar – 27.8 % Quartz – 14.8 % |
GLAZE 2: Green Colour Recipe: 0.07 CaO 0.36 ZnO 0.31 Al2O3 2.53 SiO2 0.26 TiO 0.18 K2O 0.14 Na2O Batch: Cobalt Oxide – 2 % ZnO – 11 % TiO – 8 % Potash Feldspar – 28 % Quartz – 15 % |
GLAZE 3: Red-Brown Colour Local glaze: Granite dust |
Results and discussion
The results of the various investigations are presented below. Table two gives the results of the sieve analysis of the clay sample.
Table 2. Sieve analysis of Otukpo Clay
|
Composites |
Percentage |
|
Clay |
82.48 |
|
Silt |
16.65 |
|
Sand |
1.77 |
The sieve analysis result, Table 2 showed that colloidal clay present was 82.4%, while 16.7% silt and 1.77% sand were obtained.
Results of the chemical analysis of the clay are given in Table 3.
Table 3. Chemical analysis of Otukpo Clay
|
Oxides |
Composition (%) |
|
SiO2 |
45 |
|
Al2O3 |
48 |
|
Fe2O3 |
1.3 |
|
CaO |
0.37 |
|
Na2O |
0.56 |
|
L.O.I |
6.3 |
The chemical analysis result in Table 3, showed that clay sample contains 45% SiO2, 48% Al2O, 0.37% CaO, 1.3% Fe2O3, 0.56% Na2O and rest were carbonaceous matter. These implies that the sample was made up of 89.76 % clay (kaolinite), 4.73% soda feldspar, 0.66% limestone, 1.3% Fe2O3 and 3.55% of other components. From the analysis results, it was observed that the clay has high alumina content which is a good refractory property.
Results of plasticity test carried out on the clay sample are presented in Table 4.
Table 4. Results of plasticity test
|
Sample |
Wet Rolls |
On Drying |
Performance |
|
A |
Endure all rolls |
No crack seen |
Very good |
|
B |
Endure all rolls |
No crack seen |
Very good |
|
C |
Endure all rolls |
No crack seen |
Very good |
|
D |
Endure all rolls |
No crack seen |
Very good |
|
E |
Roll with slight cracks |
Minor cracks seen |
Fair |
|
F |
Roll with slight cracks |
Minor crack seen |
Fair |
|
G |
Endure all rolls |
No crack seen |
Good |
|
H |
Endure all roll |
No crack seen |
Good |
|
I |
Endure all roll |
No crack seen |
Good |
|
J |
Endure with slight cracks |
Minor cracks seen |
Fair |
The plasticity test result from Table 4 showed that sample A, B and C are very plastic. Although sample D, G, H and I endure all rolls and no crack was seen, they were not as plastic as sample A, B, and C during forming. Sample E, F, and J were fairly plastic hence generated minor cracks when they were rolled.
Table 5 and Figure 1 illustrate the results of dry, fired and total shrinkages of the tile produced.
Table 5. Shrinkage test result
|
Sample |
Plastic length (cm) |
Dry length (cm) |
Biscuit length (cm) |
Glost length (cm) |
Dry shrinkage (%) |
Fired shrinkage (%) |
Total shrinkage (%) |
|
A |
10.00 |
8.80 |
8.70 |
8.40 |
12.00 |
4.55 |
16.00 |
|
B |
10.00 |
9.00 |
8.90 |
8.50 |
10.00 |
5.56 |
15.00 |
|
C |
10.00 |
9.00 |
9.00 |
8.90 |
10.00 |
1.11 |
11.00 |
|
D |
10.00 |
9.10 |
9.10 |
9.00 |
9.00 |
1.10 |
10.00 |
|
E |
10.00 |
9.20 |
9.20 |
9.10 |
8.00 |
1.09 |
9.00 |
|
F |
10.00 |
9.50 |
9.50 |
9.40 |
5.00 |
1.05 |
6.00 |
|
G |
10.00 |
9.30 |
9.25 |
8.90 |
7.00 |
4.30 |
11.00 |
|
H |
10.00 |
9.40 |
9.30 |
8.90 |
6.00 |
5.32 |
11.00 |
|
I |
10.00 |
9.20 |
9.10 |
8.70 |
8.00 |
5.44 |
13.00 |
|
J |
10.00 |
9.20 |
9.10 |
8.70 |
8.00 |
5.44 |
13.00 |
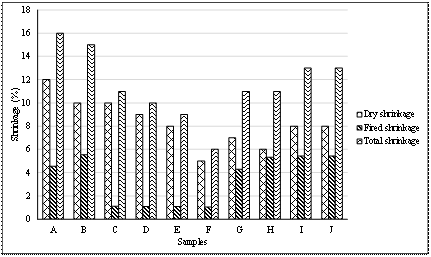
Figure 1. Chart of shrinkage test results
The shrinkage test result in Table 5 and Figure 1, showed that the total shrinkage of sample A and B which were 16 % and 15 % respective were higher than the highest shrinkage of 13 % recommended for stoneware made through plastic forming [13,14], while rest of the samples had total shrinkages ranging from 6 % to 13 %. However, the fired shrinkages of samples A and B were 4.55 % and 5.56 % respectively; this showed that the formulation would result in an acceptable shrinkage if dry pressing were used instead of plastic forming. Samples containing quartz resulted in lower fired shrinkages (1.05 % – 1.11 %) compared to the other sample. This is likely because quartz is non plastic and has lower firing shrinkage.
Results of the color test for the bisque and fired tiles are presented in Table 6.
Table 6. Color test result
|
Sample |
Green ware color |
Bisque ware |
Glost ware |
|
A |
Cream |
Brick red |
Red-brown |
|
B |
Cream |
Brick red |
Tan |
|
C |
Dark yellow |
Brick red |
Red-brown |
|
D |
Dark yellow |
Brick red |
Light-orange |
|
E |
Light yellow |
Brick red |
Cream |
|
F |
Light yellow |
Brick red |
Faded pink |
|
G |
Brick red |
Brick red |
Brick red |
|
H |
Brick red |
Brick red |
Red-brown |
|
I |
Dark spotted cream |
Brick red |
Tan |
|
J |
Dark spotted Brick red |
Brick red |
Red-brown |
From the color test result in Table 6, it was observed that the fired color of the ware range from Cream, pink, tan to red-brown due to the Fe3O2 content of the clay. The variation in color was caused by the reduction in percentage Fe3O2 as a result of addition of other components of the various samples.
Table 7 and Figure 2 give the results of water absorption test carried out on the tiles developed.
Table 7. Results of water absorption test
|
Sample |
Dry weight (g) |
Saturated weight (g) |
Water absorption (%) |
|
A |
40.40 |
41.61 |
3.00 |
|
B |
20.10 |
20.91 |
4.03 |
|
C |
34.60 |
38.52 |
11.33 |
|
D |
30.40 |
34.02 |
11.91 |
|
E |
30.20 |
34.33 |
13.68 |
|
F |
23.00 |
26.69 |
16.04 |
|
G |
24.30 |
26.36 |
8.48 |
|
H |
18.80 |
20.07 |
6.76 |
|
I |
15.50 |
17.39 |
12.19 |
|
J |
22.30 |
26.42 |
18.48 |
The water absorption test in table 7 and Figure 2, showed that sample A which has 100% clay has the lowest absorption of 3.00 % and absorption property of samples increase with increase in other raw materials present. In the case of sample I and J, the absorption was very high as introduced filler burnt off leaving pores.
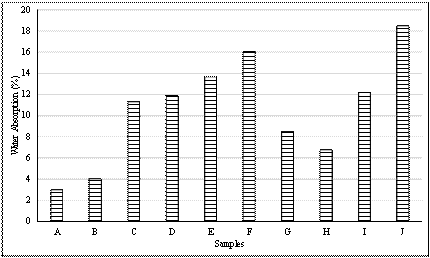
Figure 2. Water absorption chart
Samples C, D, E and F containing 20 %, 20 %, 20 % and 40 % quartz respectively had water absorptions of 11.33 %, 11.91 %, 13.680% and 16.04 % respectively. It was obvious that the increase in quartz content increased the absorption of the tile. This was likely because the free silica did not fuse completely at 1200 °C. Water absorption of sample A fell within the vitreous category, samples B, H were found to be semi-vitreous while the rest of the samples were non vitreous ANSI 137.1-2012.
Results of acid resistance test of the tile are given in Table 8.
Table 8. Acid resistance test results
|
Sample |
5 ml of 1.0 M H2SO4 (g) |
5 ml of 98 % conc. H2SO4 (g) |
Remark |
|
A |
2.0 |
2.0 |
Resistance |
|
B |
2.0 |
2.0 |
Resistance |
|
C |
1.9 |
2.0 |
Resistance |
|
D |
2.0 |
2.0 |
Resistance |
|
E |
2.0 |
2.0 |
Resistance |
|
F |
2.0 |
2.0 |
Resistance |
|
G |
2.0 |
2.0 |
Resistance |
|
H |
2.0 |
2.0 |
Resistance |
|
I |
2.0 |
2.0 |
Resistance |
|
J |
2.0 |
2.0 |
Resistance |
From the acid resistance result in Table 8, it was observed that the formulations were all resistive to acid attack. Hence, are said to be resistive to corrosion or chemical attacks.
Results of compressive strength and modulus of rupture test conducted on the tiles developed are shown in Tables 9 and 10 respectively, and illustrated in Figure 3.
Table 9. Compressive strength test result
|
Sample |
Length (cm) |
Breath (cm) |
Area (cm2) |
Force (KN) |
Mass (kg) |
C.C.S (kg/cm2) |
|
A |
2.00 |
1.70 |
3.40 |
16.4 |
1640 |
482 |
|
B |
2.60 |
2.00 |
5.20 |
18.0 |
1800 |
346 |
|
C |
1.80 |
2.10 |
3.78 |
12.0 |
1200 |
317 |
|
D |
2.00 |
1.90 |
3.80 |
7.8 |
780 |
205 |
|
E |
2.00 |
1.90 |
3.80 |
7.8 |
780 |
190 |
|
F |
1.95 |
1.90 |
3.71 |
4.4 |
440 |
119 |
|
G |
1.70 |
1.75 |
2.97 |
9.0 |
900 |
303 |
|
H |
1.95 |
1.95 |
3.8 |
17 |
1700 |
447 |
|
I |
1.95 |
2.00 |
3.9 |
16 |
1600 |
410 |
|
J |
2.2 |
1.74 |
3.8 |
9.7 |
970 |
255 |
Table 10. Modulus of rupture test result
|
Sample |
Length, L, (cm) |
Breath, b (cm) |
Applied load, F (kg) |
M = FL/4 (kg/cm) |
I = bt3/12 (cm4) |
J = 3FL/2bt3 (kg/cm2) |
|
A |
5.2 |
1.8 |
55.8 |
72.54 |
0.0768 |
487.5 |
|
B |
7.5 |
1.8 |
44.3 |
83.06 |
0.0768 |
432.6 |
|
C |
8.3 |
1.8 |
32.5 |
67.44 |
0.0768 |
351.2 |
|
D |
6.2 |
1.9 |
39.5 |
61.23 |
0.0811 |
302.1 |
|
E |
4.2 |
1.9 |
55.1 |
57.86 |
0.0811 |
285.5 |
|
F |
6.2 |
1.9 |
33.0 |
51.15 |
0.0811 |
252.4 |
|
G |
8.3 |
1.8 |
41.8 |
86.74 |
0.0768 |
451.8 |
|
H |
7.5 |
1.8 |
44.2 |
82.88 |
0.0768 |
431.6 |
|
I |
7.5 |
1.8 |
37.8 |
70.88 |
0.0768 |
369.1 |
|
J |
7.5 |
1.8 |
36.0 |
67.50 |
0.0768 |
351.6 |
|
NB. t = 0.8 cm, M = maximum bending moment, I = moment of inertia of cross section, J = modulus of rupture, bending strength or fracture strength |
||||||
The compressive strength and the modulus of rupture results in Tables 9 and 10 respectively and Figure 3 showed that all the samples have good mechanical strength. The samples withstood load above 250 lb (113.3 kg) which is the specification by America National Standard Institute (ANSI A137.1-2012). It was observed that the strength of the tiles increase with increase in clay content. Sample A with 100 % clay had the highest values of 482 kg/cm2 and 487.5 kg/cm2 for cold crushing strength and modulus of rupture respectively. The modulus of rupture also tends to increase with the increase in grog content. These occurred because the clay has high silica content which together with alkali metal presence results in glassy fusion which in turn results in high compressive strength [15, 16].
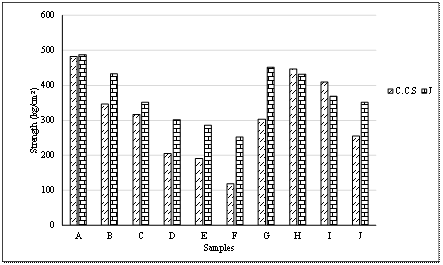
Figure 3. Compressive strength and modulus of rupture chart
Results of bulk density test for the tiles are illustrated in Table 11 and Figure 4.
Table 11. Bulk density result
|
Sample |
Dry weight Wa (g) |
Weight in water Wb (g) |
Weight in air Wc (g) |
Bulk Volume VB (cm3) |
Bulk density DB (g/cm3) |
|
A |
45.5 |
29.5 |
49.2 |
19.7 |
2.31 |
|
B |
34.4 |
22.0 |
37.9 |
15.9 |
2.16 |
|
C |
43.3 |
29.5 |
49.7 |
20.2 |
2.14 |
|
D |
51.0 |
34.5 |
58.7 |
24.2 |
2.11 |
|
E |
44.3 |
28.0 |
51.5 |
23.5 |
1.89 |
|
F |
53.1 |
35.0 |
62.3 |
27.3 |
1.95 |
|
G |
46.6 |
32.0 |
53.7 |
21.7 |
2.05 |
|
H |
49.7 |
33.0 |
55.8 |
22.8 |
2.18 |
|
I |
32.1 |
18.5 |
39.3 |
20.8 |
1.74 |
|
J |
34.9 |
22.0 |
43.5 |
21.5 |
1.62 |
NB. Density of water, D = 1 g/cm3
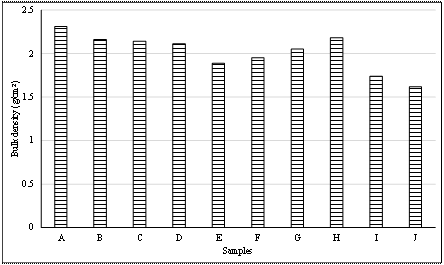
Figure 4. Bulk density result
Results of the thermal resistance test of the tiles are presented in Table 12.
Table 12. Results of thermal shock resistance
|
Sample |
First cycle |
Second cycle |
Third Cycle |
|
A |
No crack seen |
No crack seen |
No crack seen |
|
B |
Slight cracks at the back |
Crack did not expand |
No crack seen |
|
C |
No crack seen |
No crack seen |
No crack seen |
|
D |
No crack seen |
No crack seen |
No crack seen |
|
E |
No crack seen |
No crack seen |
No crack seen |
|
F |
No crack seen |
No crack seen |
No crack seen |
|
G |
No crack seen |
No crack seen |
No crack seen |
|
H |
No crack seen |
No crack seen |
No crack seen |
|
I |
No crack seen |
No crack seen |
No crack seen |
|
J |
No crack seen |
No crack seen |
No crack seen |
The bulk density (Table 11 and Figure 4) of the sample showed an increase with increased clay content. Samples I and J with 20% fiber had the least bulk densities of 1.74 g/cm3 and 1.62 g/cm3. This is due to the formation of pores left by he burnt fiber. Thermal shock resistances of all the samples were acceptable. Sample B, however had slight cracks at the back which closed up after the third heating cycle. This is likely due to the fusion of the clay as a result of prolonged heating [16]. All the glazes formulated and applied to the samples fused and yielded good results.
Conclusion
Good quality tiles have been produced from Otukpo clay by plastic forming. Although the total shrinkage of the tiles from the 100 % clay sample (sample A) was high, it did not result into cracks after firing and it had good strength and water absorption properties. Furthermore the excessive shrinkage was corrected by the addition of grog and quartz. Generally the tiles produced will fit into many areas of tile applications.
References
1. Matthew G. O., Fatile B. O., Characterization of vitrified porcelain tiles using feldspar from three selected deposits in Nigeria, Research Journal of Recent Sciences, 2014, 3(1), p. 67-72.
2. Luz A. P., Ribeiro S., Use of glass waste as a raw material in porcelain stoneware tile mixtures, Ceramics International, 2007, 33, p. 761-765.
3. Nezahat E., Arife Y., Characterization of porcelain tile bodies with Colemanite waste added as a new sintering agent, Journal of Ceramic Processing Research, 2009, 10(4), p. 414-422.
4. Abadir M. F., Sallam E. H., Bakr I. M., Preparation of porcelain tiles from Egyptian raw materials, Ceramics International, 2002, 28 (3), p. 303-310.
5. Peter W. O., Stefan J., Joseph K. B., Characterization of feldspar and quartz raw materials in Uganda for manufacture of electrical porcelains, Journal of Australia Ceramic Society, 2006, 41(1), p. 29-35.
6. Gennaro R., Cappelletti P., Cerri G., Gennaro M., Dondi M., Guarini G., Langella A. and Naimo D., Influence of zeolites on the sintering and technological properties of porcelain stoneware tiles, Journal of the European Ceramic Society, 2003, 23(13), p. 2237-2245.
7. Carbajal L., Rubio-Marcos F., Bengochea M. A., Fernandez J. F., Properties related phase evolution in porcelain ceramics, Journal of European Ceramics Society, 2007, 27, p. 4065-4069.
8. Mclaren M. G., Ceramic, The Encyclopedia Americana, Vol. 6, Grolier Incorporated, Dunbury, USA, 2001, pp. 195-196.
9. Bouyoucos G. J., The hydrometer as a new and rapid method for determining the colloidal content of soils. Soil Science, 1927, 23, p. 319-331.
10. Agboola J. B., Abubakre O. K., Investigation of appropriate refractory material for laboratory electric resistance furnace, Leonardo Journal of Science, 2009, 8(14), p. 235-243.
11. Rhodes D., Clay and glaze for the potter, Chilton Book Company, Radnor, Pennsylvania, London, 1996.
12. Conrad J. W., Ceramic formulas: The complete compendium: A guide to clay, glaze, enamel, glass, and their colours, MacMillan Publishing Co. Inc., New York, 1974.
13. Deer W. A., Howie R. A., Zussman J., An introduction to the rock-forming-minerals, 2nd Edition, Longman group (FE) Limited, Hong Kong, 1992, p. 391-470.
14. Mclaren M. G., Ceramic, The Encyclopedia Americana, vol. 6, Grolier Incorporated, Dunbury, USA, 2001, p. 195-196.
15. Nnuka E. E., Agbo J. E., Evaluation of the refractory characteristics of Otukpo Clay deposit, N.S.E Technical Transaction, 2000, 35(1), p. 32-39.
16. Ibitoye S., Alo O., Adaptation of Odolewu clay for use as refractory material, International Journal of Scientific & Engineering Research, 2014, 5(4), p. 837-844.