Corrosion inhibition of Na2CO3 on low carbon steel in Ureje seawater environment
Daniel Toyin OLORUNTOBA
Metallurgical and Materials Engineering Department, Federal University of Technology, P.M.B. 704, Akure. Ondo State, Nigeria
E-mails: dtoloruntoba@futa.edu.ng; oloruntobadt@yahoo.com
* Corresponding author, phone: +2348033636164
Received: January 12, 2016 / Accepted: July 14, 2016 / Published: July 31, 2016
Abstract
Combating the corrosion effect on the steel structure of car body from sea water of local areas in Nigeria is the thrust of this research. Low carbon steel corrosion inhibition in seawater environment was investigated with sodium carbonate (Na2CO3). Steel samples were immersed in 400 dm3 seawater from Ureje community area in Ado Ekiti Nigeria with 0.01M and 0.1M Na2CO3 as the inhibitor. The electrochemical method of corrosion measurement was adopted for the study. The electrochemical behaviour was investigated with PGSTAT 101 Metrohm Potentiostat using Saturated Ag/AgCl Electrode as the counter electrode. The polarization curves were fixed at ±1.5 mV. Potentiodynamic polarization parameters obtained gave inhibition efficiency of Na2CO3 on the low carbon steel as 40.4% and 98.6% at 0.01M and 0.1M respectively. Thus, sodium carbonate formed an active corrosion inhibitor for low carbon steel in Ureje seawater environment. It could be introduced into the seawater in the stated concentration for a cleansing of steel structure in the car body.
Keywords
Seawater; Steel; Sodium carbonate; Inhibitor; Corrosion; Electrochemical; Potentiostat; Efficiency
Introduction
Low carbon steel is the least expensive type of steel and commonly used for construction purposes. Applications of steel include: fence wire, auto body, galvanized sheet, storage tanks, large pipe, and various parts of building, bridges and ships [1]. Steel is highly susceptible to corrosion in chloride or seawater environment [2]. Corrosion is defined amongst other definitions as an attack on metallic and non-metallic materials by reaction with its environment [3]. Each metallic substance is endowed with a certain natural energy level or potential, in that metals occupying different energy levels or potentials coupled together, current flows to the direction of higher potentials. The directions of positive current flow will be from the metal with the most negative potential through the soil to that more positive [4]. Corrosion will occur at the point where positive current leaves the metal surface. A dry cell battery is one example of a corrosion cell [4].
When iron or steel is exposed to atmospheric oxygen in the presence of water, the common rust processes take place. Degradation of metals forms ferric rust, a red-brown Compound; that is a sure sign of electrochemical oxidation of the underlying metal [5]. Metals, except platinum and gold, will corrode in an oxidizing environment, forming compounds such as oxides, hydroxides and sulphides as reflected in equation 1-3 [6].
|
|
(1) |
|
|
(2) |
|
|
(3) |
Seawater typically contains about 3.5% sodium chloride, although the salinity may be weakened in some areas by dilution with fresh water or concentrated by solar evaporation in others [7]. Seawater is normally more corrosive than fresh water because of the higher conductivity and the penetrating power of the chloride ion through surface films on a metal. The rate of corrosion is controlled by the chloride content, oxygen availability, and the temperature [7]. Corrosion prevention has been partially achieved by material selection, cathodic protection, surface protection (coating), and or by the use of inhibitors [8-11]. In a case there is no way for reconstruction or surface treatment steel parts the option of inhibitor which could either be organic or in-organic would be used. Most cars in the South Western Nigeria are commonly washed with water from pond or short flowing rivers.
The work investigated the use of Sodium Carbonate as inhibitor in the collection of the short flowing river for the washing of a car aiming to reduce corrosion of the body especially in the intricate parts.
Material and method
Low carbon steel sample was obtained from part of a car. The spectrometric spark analysis of the sample was done to determine the chemical composition. Qualitative analysis of Ureje seawater at Ado Ekiti Nigeria was also carried out for chemical constituents. The spectrometric and the qualitative analysis results are shown in Tables 1 and 2.
Table 1. Spectrometric analysis of the low carbon steel of the car body
|
Element |
Fe |
C |
Si |
Mn |
P |
S |
Cr |
Ni |
|
Composition (%) |
98.61 |
0.1787 |
0.0863 |
0.2714 |
<0.000 |
0.0308 |
0.0920 |
0.2211 |
|
Element |
Mo |
Cu |
Al |
Ti |
V |
Co |
Nb |
W |
|
Composition (%) |
<0.014 |
0.0555 |
0.1041 |
0.0111 |
0.0306 |
0.0526 |
0.0965 |
<0.031 |
|
Element |
Sn |
|
||||||
|
Composition (%) |
0.0093 |
|
||||||
Table 2. Qualitative analysis of Ureje river sample Ado-Ekiti
|
S/N |
Parameters |
Ureje River |
|
1 |
pH (25°C) |
8.40 |
|
2 |
Conductivity (µs/cm) |
227 |
|
3 |
Total solid (mg/L) |
1.6 |
|
4 |
Dissolved solid (mg/L) |
1.0 |
|
5 |
Suspended solid (mg/L) |
0.61 |
|
6 |
Phenolphthalein alkalinity (mg/L) |
ND |
|
7 |
Total alkalinity (mg/L) |
240 |
|
8 |
Acidity (mg/L) CaCO3 |
30 |
|
9 |
Total Hardness (Mg/L) |
120 |
|
10 |
Ca+2 (mg/l) |
20.04 |
|
11 |
Mg+2 (mg/l) |
17.02 |
|
12 |
Cl- (mg/l) |
61.54 |
|
13 |
SO4-2 (mg/L) |
1564 |
|
14 |
NO3- (mg/L) |
0.01 |
|
15 |
PO4-3 |
0.03 |
Electrochemical measurements were performed using a typical three-compartment glass cell (Autolab Pgstat204 model) at the Metallurgical Laboratory of the Federal University of Technology Akure. The experimental set up consists of the steel specimen as the working electrode (1 cm2), saturated silver/silver chloride as reference electrode, and a platinum foil as a counter electrode.
Prior to carrying out the experiments, the working electrode was abraded with 1200 grit emery paper, cleaned with distilled water in acetone and dried with jets of hot air. Autolab Pgstata204 model linked with personal computer running on NOVA software was used for data collection and data processing.
Before potentio-dynamic polarization measurement, open-circuit corrosion potential (OCP) measurements were carried out for 30 minutes that was proved to be sufficient to attain a stable value of Ecorr. The tafel polarization study was carried out from cathodic potential of −250 mV to an anodic potential of +250 mV with a scan rate 1.0 mVs−1 to determine the current density (Icorr), corrosion rate and anodic tafel slope. 0.01M and 0.1M Na2CO3 concentrations were used as inhibitor in the Ureje seawater environment.
A flowchart of the operations of inhibitor of steel sample from car body in Ureje seawater environment with sodium carbonate is shown as:
Corrosion inhibition efficiency IE was calculated using the equation (3):
|
|
(3) |
where: I'corr = corrosion current density value with inhibitor, Icorr = corrosion current density value without inhibitor. Model equation from correlation terms from ANOVA is illustrated by equation 4:
|
Corrosion rates = +0.20923 -0.093482*IC -0.093482*PP |
(4) |
where, IC= Inhibitor Concentration, PP= Polarization Potential
Results and discussion
Intricate parts or areas of regression usually harbour the water and residue from the wash thereby leading to the corrosion of the car body as shown in Figure 1.

Figure 1. Photograph image of corroded car bonnet
Result showed a positive displacement of polarization potential, blank seawater having polarization potential of -0.855 V vs (Ag/AgCl) while the inhibited seawater have -0.680 V vs (Ag/AgCl) and -0.478 V vs (Ag/AgCl) at 0.01M and 0.1M Na2CO3 concentrations respectively.
The least corrosion current and highest polarization resistance of 4.57E-7 A/cm2 and 4270.0Ω respectively was obtained for steel sample immersed in Ureje seawater with 0.01M sodium carbonate inhibitor shown in Figure 2 and Table 3. The plot of the data obtained on potentio-dynamic and current density is shown in Figure 2. Polarisation resistance and corrosion rates data are shown in Table 3. It can be observed in Figure 2 that the highest corrosion density (3.26E-5 A/cm2) was recorded by the sample without inhibitor that is, the blank.
Table 3. Polarization parameters of steel samples in Ureje River with and without sodium carbonate as inhibitor
|
Ureje River |
Ecorr(V) |
Icorr(A/cm2) |
Corrosion rate (mg/mm2/year) |
Polarization resistance(Ω) |
Inhibitor Efficiency (%) |
|
blank |
-0.855 |
3.26E-5 |
0.37924 |
1824.3 |
- |
|
0.01M |
-0.680 |
1.95E-5 |
0.22618 |
3503.3 |
40.4 |
|
0.1M |
-0.478 |
4.57E-7 |
0.005314 |
4270.0 |
98.6 |
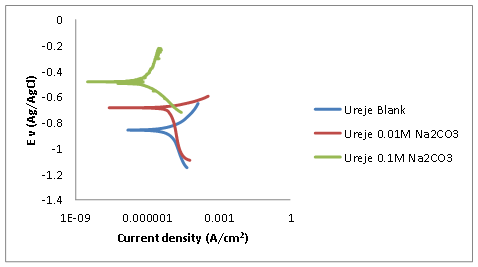
Figure 2. Polarization curves of low carbon steel immersed in Ureje seawater with different concentrations of sodium carbonate as inhibitor
The chloride content (61.54 (mg/l) Cl-) with sulphide combination 1564 (mg/l) SO4-2 (Table 2) in the Ureje seawater could be responsible for the corrosion parameters of the blank solution.
As observed in Figure 2 and Table 3, the higher the concentrations of the inhibitor, the lower the current density and the corrosion rates of the steel samples. That correlates with the findings of [8] that at high concentration, anions may either become inhibitive or appear in such a way as to plug any pores in a passive film.
However, the corrosion inhibition efficiency of 40.4 % and 98.6% recorded for 0.01M and 0.1M Na2CO3 concentrations respectively indicates that a protective film is formed on the metal surface. The passivation of the mild steel may be caused by the formation of Fe2O3 and Fe3O4 [12].
Protective films formed on the metal surface will cause linear polarization resistance (LPR) value to increase and decrease in corrosion current values [5], and [9]. An inhibitor can be classified as cathodic or anodic type if the shift of corrosion potential in the presence of the inhibitor is more than 85 mV with respect to that in the absence of the inhibitor [10] in this case, 377 mV shifts was achieved for the 0.1M Na2CO3 concentration. Hence, sodium carbonate can be classified as an anodic type of inhibitor.
Table 1 gives the composition of the steel as plain carbon without sufficient alloy elements to combat corrosion in the high chlorinated seawater. Hence, corrosion inhibition achieved for the steel in the used environment is as a result of the Sodium carbonate salt introduced. Sodium carbonate salt is practically effective to combat the corrosion of car body where the water for washing contains high chloride and sulphide ions. It is necessary to investigate further the amount needed in a larger volume of seawater.
The interaction graph of the corrosion behaviour of inhibitor and polarization characteristics of steel sample immersed in Ureje seawater environment displayed in Figure 3.
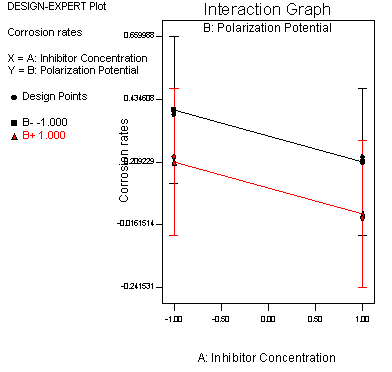
Figure 3. Interactive plot of Na2CO3 inhibitor concentration and polarization potential of steel sample immersed in Ureje River Ado-Ekiti
When the current density of cathodic polarization exceeds a certain threshold, the electrode enters the passive/active transition region as the polarization potential moves to a more negative value [13]. The steel will experience passivation at more positive potential as well as at the higher concentration of the inhibitor. Polarized potential will enhance the performance of the inhibitor in the chloride environment. Result of the polarization curves shows a similar trend with the findings that corrosion current density decreases after tannic acid is added to mild steel in seawater environment [14].
Figure 4 portrays the 3-D map of the variables interaction on corrosion behaviour of the steel sample in Ureje seawater. The best interaction is achieved at plane 1 1 while the plane -1 -1 shows the negative value of the interaction on corrosion rates. Plane 1 1 indicated that higher positive potentials and the higher concentration of the inhibitor favour corrosion protection of the steel sample in Ureje seawater environment.
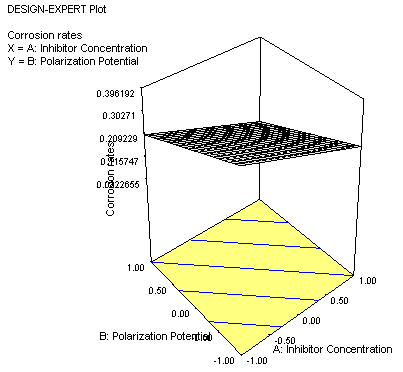
Figure 4. 3-D map of the modelling process showing the interaction between the polarization potential and inhibitor concentration
Conclusions
1. Sodium carbonate acted as an anodic inhibitor of low carbon steel in Ureje Seawater environment.
2. The higher the inhibitors concentration, the greater the corrosion inhibition efficiency of the sodium carbonate on low carbon steel immersed in the seawater from Ureje River of Ado-Ekiti.
3. The inhibition efficiency increases with increasing inhibitor concentrations to attain a maximum value of 98.6% at 0.1M sodium carbonate inhibitor.
Acknowledgements
Mr. F.O Ogundeji is acknowledged for data collection.
References
1. Brabdt A.D., Jarius C.W., Metallurgy fundamentals, Illinois United States, the Goodheart-Willcox Company Inc, 2005.
2. Otunniyi I. O., Oloruntoba D.T., Suitability of Structural Aluminium Profiles as Sacrificial Anode for Carbon Steel, Leonardo Electronic Journal of Practices and Technologies, 2012, 11(21), p. 62-72.
3. Einer B., Corrosion and protection- Engineering materials and processes, British Library Cataloguing in Publication Data, 2000, pp. 70.
4. Bushman, B. J., Corrosion and Cathodic Protection Theory. Available at: http://www.bushman.cc/pdf/corrosion_theory.pdf (accessed November 14, 2015).
5. Mary C., Anbarasi S. R., Vijaya N., Manivannan M., Shanthi T., Corrosion inhibitor of ion pair reagent-Zn2+ system, Open Corrosion Journal, 2012, p. 1-7.
6. Moudgil H. K., Textbook of Physical Chemistry Second Edition, Delhi, PHI Learning Private Limited, 2015.
7. Pierre R. R., Corrosion Basics-An Introduction Second Edition, Houston TX, NACE International, 2006.
8. Trethewey K. R., Chamberlain J., Corrosion for Science and Engineering. 2nd edition, England, Edinburgh Gate Harlow Essex CM20 2JE, 1995.
9. Oloruntoba D.T., Popoola A.P.I., Effect of coating on induced thermal and tensile stress on the fracture of exhaust pipe material, Engineering Failure Analysis Elsevier, 2015, 56, p. 562-572.
10. Shalabi K., Abdallah Y. M., Hala M. H., Fouda A.S., Adsorption and corrosion inhibition of atropa belladonna extract on carbon steel in 1M HCl solution, International journal of electrochemical science, 2014, p. 1469-1470.
11. Oloruntoba D. T., Corrosion Inhibition of Water Hyacinth on 1014 Steel in a Chloride Environment, Caspian Journal of Applied Sciences Research, 2013, 2(2), p. 6-16.
12. Fraunhofer J. A., The polarization behaviour of mild steel in aerated and de-aerated 1M NaHCO3, Corrosion Science, 1970, 10(4), p. 245-251.
13. Hu Q., Qiu Y. B., Guo X. P., Huang J. Y., Crevice corrosion of Q235 carbon steels in a solution of NaHCO 3 and NaCl, Corrosion Science, 2010, 52(4), p. 1205-1212.
14. Qian B, Hou B., Zheng M., The inhibition effect of tannic acid on mild steel corrosion in seawater wet/dry cyclic conditions, Corrosion Science, 2013, 72, p. 1-9.