Exergy analysis of waste emissions from gas flaring
Olawale Saheed ISMAIL*, Kazeem TAJUDEEN
Department of Mechanical Engineering, University of Ibadan, Ibadan, Oyo State, Nigeria
E-mails: os.ismail@ui.edu.ng; tajqas@gmail.com
* Corresponding author: Phone: +234 8023371570
Received: June 2, 2016 / Accepted: July 17, 2016 / Published: July 31, 2016
Abstract
Gas flaring produces a stream of waste gases at high temperature and pressure which contains carbon monoxide, Hydrogen Sulphide etc. The resultant effect of which is detrimental to our planet and, consequently, to the life of both the living and the non-living things. It’s well known that gas flaring contributes in no small measure to the global warming. Exergy analysis is applied in this work to analyze waste emissions from gas flaring so as to have a model through which impact of gas flaring can be measured. The study considers both the thermo-mechanical exergy and the chemical exergy of these gases. Relevant data on gas flaring activities in the Niger-Delta region of Nigeria between the periods of fifteen (15) years was obtained from the Nigerian National Petroleum Corporation (NNPC). A computer program (Exergy Calculator) was developed based on the equations generated in the Model. Exergy associated with gas flaring activities in Nigeria between the periods of 1998 through 2012 was calculated. The results show that 1 mscf (in thousand cubic feet) of flared gases generate 0.000041 MWh of energy leading to a value of 440158.607 MWh of energy for the period under review.The analysis provides important conclusions and recommendations for improving oil platforms operationsin in order to safeguard the environment, health of the populace, and maximize recovered exergy from gas flaring.
Keywords
Exergy; Gas Flaring; Exergy Calculator; Flare Stack; Associated Gases; Environmental Degradation; Niger-Delta
Introduction
The phenomenon of gas flaring and associated environmental degradation is well known. This has been generating lots of debates over time. One will wonder, why will oil companies allow all of these gases that should be of economic values to be wasted as it is presently the practice? Why has it been difficult for the Oil Companies to tap into the economic benefits offered by proper usage of the flared gases? Better still, the question could be poised:” are there enough benefits (environmental or commercial) in these gases to justify the huge capital investment that would have to be made in them?” Therefore, there is need to carry out exergy analysis on the waste emissions vis-à-vis their economic values and effects on the environment.
Gas flaring is the burning of natural gas that is associated with crude oil when it is pumped up from the ground. In petroleum-producing areas where insufficient investment is made in infrastructure to utilize natural gas, flaring is employed to dispose of these associated gases. This produces emissions which degrade the enivironment and are harmful to both living things and non living things, reducing it as much as possible should be the concern to all [1]. Various gases emitted includes Carbon Dioxide (CO2), Methane (CH4), Nitrous Oxide (N2O), Hydrofluorocarbons (HFCs), Perfluorocarbons (PFCs), Sulphurhexafluoride (SF6), sulphur dioxide (SO2), hydrogen sulphide (H2S), unburned fuel, particulate soot and other by-products of combustion [2-6], effort should be made to reduces these emissions [7] since there is a threshold which can be tolorated by habitats living in the environment [8].
Exergy analysis is applied in this work to analyze waste emissions from gas flaring inorder to have a model through which its impact can be measured. Exergy analysis has been used over the years for diffent applications [9-14].
Exergy analysis is based upon the second law of thermodynamics, which stipulates that all macroscopic processes are irreversible. Every such irreversible process entails a non-recoverable loss of exergy, expressed as the product of the ambient temperature and the entropy generated. Some of the components of entropy generation can be negative, but the sum is always positive [15].
A complete set of updated values of chemical exergies of elements at standard conditions (298.15K and 1 atm) has been tabulated to easy regious caculationin in exergy analysis [16]. Their values will be adopted for this work.
Material and method
Exergy, basically, is of two types: (1) Thermo-mechanical Exergy and (2) Chemical Exergy.
Thermo-mechanical exergy is the exergy associated with the conversion of thermal energy to mechanical energy and disregards any mixing and chemical reactions. Depending on the type of system involved, the following types of thermo-mechanical exergy exist:
Potential energy which is expressed as:
![]() (1)
(1)
where PEexergy: potential exergy (kJ), m: mass of gas (kg), z: elevation above a datum (m).
Kinetic energy which is expressed as:
![]() (2)
(2)
where KEexergy: kinetic exergy (kJ), c: velocity of the gas (m/s).
Reversible and Irreversibility: Reversible work is determined from the exergy balance relations by setting the exergy destroyed equal to zero. The work, W, in that case becomes the reversible work. The irreversibility associated with gas flaring in Nigeria is 100% as all of the gases produced are flared off. Therefore,
![]() (3)
(3)
where Wuseful: useful work (kJ), Wrev: reversible work (kJ), Wsurrounding: work done on the surrounding (kJ), Psurrounding: surrounding pressure (kPa) and v2-v1: change in volume (m3).
![]() or
or ![]() (4)
(4)
where Iirreversible (= I): Irreversibility (kJ), Wrev,out: reversible work out (kJ), Wuseful,out: useful work out (kJ), Wuseful,in: useful work in (Kj), Wrev,in: Reversible work in (kJ).
The exergy of a system that is in equilibrium with its environment is zero. For the purpose of this study the state of the environment will be regarded as ‘dead state’ as practically no useful work can be derived from it. Flared gases are often ejected into the atmosphere in a state totally different from that of the environment. Hence, appreciable amount of exergy is embedded in these gases while it is being brought to equilibrium with environment. This difference in both the states of gases and environment necessitates the change (exergy) in the system.
Since Flared gases are constantly in motion and often involve transfer of masses across the system boundary which makes it to be likened to a control volume, the flow (or stream) conservation exergy equation is used.
The exergy of fixed mass (Non-flow) system is given by
![]() (5)
(5)
where Exergynonflow: Non-flow exergy (kJ), U: System Internal Energy (kJ), Usurr: surrounding internal energy (kJ), V: system volume (m3), Vsurr: surrounding volume (m3), Tsurr: surrounding temperature (K), Ssurr: entropy of the surrounding (kJ/K), S: entropy of the system (kJ/K).
But for flow system, there is an additional form of energy, called the flow energy, which is the energy, needed to maintain flow in a pipe or duct, and was expressed as:
![]() (6)
(6)
where Wflow: flow work (kJ), P: system pressure (kPa), v: specific volume of the fluid (m3/kg)
Flow work is essentially the boundary work done by the fluid on the fluid downstream, and thus the exergy associated with flow work is equivalent to the exergy associated with the boundary work. This is simply the difference between the boundary work and the work done on the environment. Noting that flow work is P·v and the work done against the atmosphere is Psurr·v; the exergy associated with flow energy can be expressed as:
![]() (7)
(7)
where Exergyflow: flow exergy (kJ).
The Total Thermo-Mechanical Exergy (Ψ) is obtained by addition of equations (5) and (7) i.e. Ψ = Exergynonflow + Exergyflow. Cumulating:
![]()
![]() (8)
(8)
Thus,
![]() (9)
(9)
where h: enthalpy of the system (kJ), hsurr: enthalpy of the surrounding (kJ).
The chemical exergy of a substance is the maximum work that can be obtained from it by taking it to chemical equilibrium with reference to the environment at constant temperature and pressure. With this definition, the environment has zero exergy but other air composition would have chemical exergy (Ech) greater than zero. Table 1 show various substances with their molar chemical exergy.
Table
1. Molar exergy, (![]() ), of pure species relative to a
reference atmosphere at P0=100 kPa, T0 =25 ºC, and 60%
RH;
), of pure species relative to a
reference atmosphere at P0=100 kPa, T0 =25 ºC, and 60%
RH; ![]()
|
Substance |
Formula (state) |
Molar fraction in ref. atm. (Xi) |
Molar exergy (Echi) [kJ/mol] |
|
Nitrogen |
N2(g) |
0.7651 |
0.66 |
|
Oxygen |
O2(g) |
0.2062 |
3.9 |
|
Water |
H2O(l) |
0.0190 |
1.3 |
|
Argon |
Ar(g) |
0.0094 |
12 |
|
Carbon dioxide |
CO2(g) |
0.0003 |
20 |
|
Carbon monoxide |
CO(g) |
NA |
275 |
|
Hydrogen |
H2(g) |
NA |
236 |
|
Methane |
CH4(g) |
NA |
831 |
|
Ethane |
C2H6(g) |
NA |
1500 |
|
Ethylene |
C2H4(g) |
NA |
1360 |
|
Acetylene |
C2H2(g) |
NA |
1265 |
|
Propane |
C3H8(g) |
NA |
2150 |
|
n-Butane |
C4H10(l) |
NA |
2800 |
|
Carbon (graphite) |
C(s) |
NA |
410 |
|
Nitrogen monoxide |
NO(g) |
NA |
89 |
|
Nitrogen dioxide |
NO2(g) |
NA |
56 |
|
Ammonia |
NH3(g) |
NA |
340 |
|
Methanol |
CH3OH(l) |
NA |
720 |
|
Ethanol |
CH3CH2OH(l) |
NA |
1400 |
|
Source: Isidoro Martinez, ‘Chemical Exergy’, 2005. [17] |
|||
The molar exergy of the pure component, at T0, P0, and if in gaseous state, is:
![]() (10)
(10)
where ![]() is
the chemical exergy of the constituent gases (kJ), Ru = 8.314J/(mol·K),
T* = 298.15K, and xio its molar fraction in
the environment, T0, P0 = surrounding temperature (K) and
pressure (Kpa) respectively.
is
the chemical exergy of the constituent gases (kJ), Ru = 8.314J/(mol·K),
T* = 298.15K, and xio its molar fraction in
the environment, T0, P0 = surrounding temperature (K) and
pressure (Kpa) respectively.
For substances not in the reference atmosphere (but with all of its different atoms represented in the environment), e.g. for CO, a chemical reaction must be set to produce it from the existing species in the environment, for example, CO2 = CO+ (1/2) O2.
For substances not in the reference atmosphere (but with all of its different atoms represented in the environment), e.g. for CO, a chemical reaction must be set to produce it from the existing species in the environment, e.g. CO2 = CO + (1/2)O2.
Therefore, the general exergy equation of any system is the combination of the thermo-mechanical exergy (flow + Non-flow) and chemical exergy.
Thus:
![]() (11)
(11)
where ![]() = General Exergy
(Total exergy) (kJ).
= General Exergy
(Total exergy) (kJ).
![]() (12)
(12)
where ![]() = Chemical exergy of the constituent gases (kJ).
= Chemical exergy of the constituent gases (kJ).
For the case of flare gases, where difference by-prodcts are involved equation 12 becomes:
![]() (13)
(13)
where ni = no of moles of constituent gases (mol)
![]() (14)
(14)
where si = entropy of constituent gases (kJ/K), hi = enthalpy of constituent gases (kJ).
But:
![]()
where Cpi: specific heat at constant pressure of constituent gases (kJ/K).
Also:
 (15)
(15)
where T1, T2: initial and final temperatures (K), P1, P2: initial and final pressures (kPa)
Hence, equation (13) becomes:
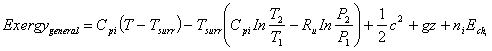 (16)
(16)
![]() (17)
(17)
where V2,i = final volume of constituent gases (m3).
The constituents of the flared gas were taken from the work of AbdulKareem [18] as shown in Table 2.
The analysis was conducted as given in Figure 1
Table 2. Percentage by volume of hydrocarbonsin flared gas in an oil station in Niger-Delta area
|
Components |
CH4 |
C2H6 |
C3H8 |
C4H10 |
C5H12 |
Others |
|
% Composition by volume |
47 |
18 |
20 |
5 |
9 |
1 |
|
Source: Abdulkareem [18] |
||||||
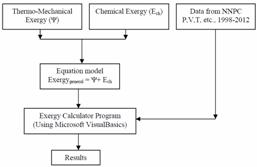
Figure 1. The algorithm of the present work
Results and discussion
Gas flaring has proven to be a major contributor to the generation of Green House Gases (GHG) which consequently has serious negative impacts on the natural environment. In as much as the modern economic development depends largely on the use of technologies driven by power generated through hydrocarbons, oil exploration will continue to play a dominant role in man’s quest for energy. Unfortunately, this process leads to flaring of associated gases (as it is presently the practice) which constitutes serious environmental challenges to our ecosystems. A computer program (Exergy Calculator) was developed (Figure 2) based on the equations generated above using Microsoft Visual Basic 6.0 Software (Working Edition).
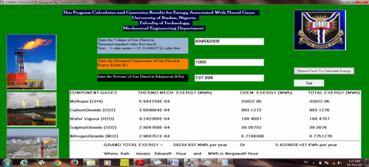
Figure 2. Interface of the Exergy Calculator developed
The program was tested and the results are consistent with the equations given. With the software developed (Figure 3), the exergy of flared gases for the period under review (i.e. 1998 - 2012) is as given in Table 3. 440,158.607 Megawatt-hour (MWh) of Exergy was generated through flared gases over the period of fifteen years. This is an enormous energy considering the fact that the average power generation in Nigeria over the period is 2,800 Megawatt-hour (MWh).
Table 3. Results of gas flared Vs. Exergy from 1998-2012
|
Years |
Volume of flared gas (mscf) |
Exergy (MWh) |
|
1998 |
834,542,305 |
34234.632 |
|
1999 |
792,247,965 |
32499.634 |
|
2000 |
882,760,070 |
36212.624 |
|
2001 |
920,905,671 |
37777.436 |
|
2002 |
744,108,035 |
30524.834 |
|
2003 |
846,013,629 |
34705.208 |
|
2004 |
886,070,556 |
36348.424 |
|
2005 |
812,332,777 |
33323.552 |
|
2006 |
799,998,368.20 |
32817.57 |
|
2007 |
789,546,171.84 |
32388.796 |
|
2008 |
631,188,574.46 |
25892.646 |
|
2009 |
824,262.75 |
33.815 |
|
2010 |
581,568,353.85 |
23857.124 |
|
2011 |
619,032,858.01 |
25393.998 |
|
2012 |
588,666,724.18 |
24148.314 |
Since there is fine attached to the volume of flared gases in Nigeria there has been allegations of underreporting by the Oil Multinationals, hence, data acquired through the use of satellite was adjudged to be more accurate in giving true picture of gas flared (Figure 2).
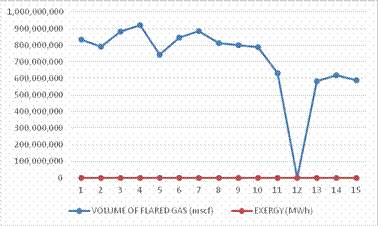
Figure 3. Graph of Gas flared vs. Exergy
With the present practice, an average of 78 % of our natural is being flared on the daily basis not minding the attendant effect of global warming. More so, Nigeria cannot afford to sit back and watch her natural resources to continue wasting in this manner of gas flaring. The should form a synergy in addressing this monumental wastage of resources and global environmental – health calamity. Gas flaring must time to act is now and all stakeholders in the energy, environmental, and health sectors stop.
Conclusions
In the course of carrying out this study it was discovered that huge amount of exergy always accompany every cubic feet of flared gas in the Niger-Delta region of Nigeria. Also, observation was made that these are useful energies that if properly harnessed should have translated into generation of several megawatt-hours (MWh) of electricity.
References
1. Ismail O. S., Umukoro G. E., Modelling combustion reactions for gas flaring and its resulting emissions. Journal of King Saud University – Engineering Sciences, 2016, 28, p. 130-140.
2. Strosher M. T., Characterization of emissions from diffusion flare systems, Journal of the Air & Waste Management Association, 2000, 50 (10), p. 1723-1733.
3. Johnson M. R., Coderre A. R., An analysis of flaring and venting activity in the Alberta upstream oil and gas industry, Journal of the Air & Waste Management Association, 2011, 61 (2), p. 190-200.
4. McEwen J. D. N., Johnson M. R., Black carbon particulate matter emission factors for buoyancy driven associated gas flares, Journal of Air & Waste Management Association, 2012, 62(3), p. 307-321.
5. Pohl J. H., Lee J., Payne R., Tichenor B. A., Combustion efficiency of flares, Combustion Science and Technology, 1986, 50(4-6), p. 217-231.
6. Umukoro G. E., Ismail O. S., Modelling emissions from natural gas flaring, Journal of King Saud University - Engineering Sciences, 2015, doi:10.1016/j.jksues.2015.08.001
7. Johnson M. R., Coderre A. R., Opportunities for CO2 equivalent emissions reductions via flare and vent mitigation: A case study for Alberta, Canada, International Journal of Greenhouse Gas Control, 2012, 8, p. 121-131.
8. Ismail O. S., Umukoro G. E., Global Impact of Gas Flaring, A Journal of Energy and Power Engineering, 2012, 4, p. 290-302.
9. Sunil Chamoli, Exergy analysis of a flat plate solar collector, Journal of Energy in Southern Africa, 2013 24(3), 8-13.
10. Bogusława Łapczyńska-Kordon, Zbigniew Ślipek, Tomasz Hebda, Exergy analysis of a bakery stove, Polish Society of Agricultural Engineering, 2013, 148 (4), p. 95-102.
11. Guiyin Fang, Lin Xing, Fan Yang and Hui Li, Exergy analysis of a dual-mode refrigeration system for ice storage air conditioning, International Journal on Architectural Science, 2005, 6(1), p. 1-6.
12. Rashad A., El Maihy A., Energy and Exergy Analysis of a Steam Power Plant in Egypt, 13th International Conference on aerospace sciences & aviation technology, asat - 13, May 26-28, 2009.
13. Mora C. H., de Oliveira Jr B. S., Environmental exergy analysis of wastewater treatment plants, Engenharia Térmica (Thermal Engineering), 2006, 5 (2), p. 24-29.
14. Adewole O. S., Ismail O. S., Performance Evaluation and Environmental Impact Assessment of Systems with Waste Exergy Emissions, International Journal of Scientific & Engineering Research, 2012, 3(7), p. 1-13. Avatlable at: http://www.ijser.org/researchpaper%5CPerformance-Evaluation-and-Environmental-Impact-Assessment-of-Systems-with-Waste-Exergy-Emissions.pdf
15. Stanek W., Szargut J., Ziebik A., Depletion of the non-renewable natural exergy resources as a measure of the ecological cost, Energy Conversion & Management, 2002, 42, p. 1149-1163.
16. Rivero R., Garfias M., Standard chemical exergy of Elements Updated, Journal of Energy 2006, 31, p. 3310-3326.
17. Isidoro Martinez, (online), ‘Chemical Exergy’. Available at http://webserver.dmt.upm.es/~isidoro/ (accessed 21/09/2015).
18. Abdulkareem A. S., Evaluation of ground level concentration of pollutant due to gas flaring by computer simulation: a case study of Niger-delta area of Nigeria, Leonardo Electron. J. Pract. Technol. 2005, 4 (6), p. 29-42.